The recently completed OPUS-2, a multicenter, randomized, double masked, placebo-controlled, parallel-arm Phase III study, examined the efficacy and safety of lifitegrast 5.0% solution in 718 adults with dry eye. Lifitegrast is a preservative-free topical solution designed to reduce inflammation caused by the interaction of lymphocyte function-associated antigen 1 and intercellular adhesion molecule-1.
| |
|
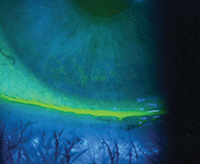
Corneal staining revealing dry eye.
|
During the OPUS-2 trial, the study agent was administered twice daily for 12 weeks in dry eye patients with a history of using artificial tears within 30 days of screening. Compared to placebo, lifitegrast showed a statistically significant improvement in patient-reported symptoms of ocular dryness (p<0.0001) over the duration of the clinical trial. However, the solution did not show statistically significant improvement in inferior corneal staining via fluorescein staining vs. the placebo subjects (p=0.6186).
The most commonly reported adverse events were dysgeusia, irritation at the site of instillation and a reduction in visual acuity. However, no serious AEs were reported.
OPUS-2 is the second in a three-part series of studies that comprise lifitegrast’s Phase III development program. In OPUS-1, the first part of the development program, lifitegrast was superior to placebo in improved inferior corneal fluorescein staining (p=0.0007), but the visual-related function of the Ocular Surface Disease Index did not achieve statistical significance.
“There’s often a real disconnect between improved pre-specified symptoms (i.e., comfort) and clinical signs, such as inferior corneal staining, with various time-points being critical,” says Joseph Shovlin, OD. “Unfortunately, the FDA seems to be holding firm to its rigid standards on any new approvals; specifically, signs of improvement clinically, such as staining, must be obtained as a pre-specified endpoint.”
Developer Shire Pharmaceuticals began the third part of the Phase III development, SONATA, in December 2012. This prospective, randomized, double-masked, placebo-controlled trial of 300 dry eye patients will evaluate safety and efficacy over one year. It should be completed by mid-2014.
“The efficacy outcomes are yet to be fully determined for lifitegrast, and the Agency assures both for safety and efficacy in the approval process,” adds Dr. Shovlin. “I’m afraid a significant hurdle remains.”


