 |
One of the hallmarks of the cornea is its avascular, transparent nature, which is a result of the precise composition and arrangement of its constituent parts. A variety of affronts—including infection, inflammation, ischemia, degeneration and loss of the stem cell barrier—can lead to the loss of this avascularity in the form of corneal neovascularization.1,2,
Over 1.4 million patients develop corneal neovascularization each year, with up to 12% of cases associated with subsequent decreased acuity as immature and abnormal vessels invade from the limbal vascular plexus, causing scarring, edema and inflammation.1,3 This invasion occurs when the habitually precise balance between pro- and anti-angiogenic factors is disturbed by an excess of pro-angiogenic factors.4
While a number of constituents promote new vessel proliferation, vascular endothelial growth factor (VEGF) is one of the key regulators of this process and, as such, has become an important target for medical therapy.5 Anti-VEGF treatments, a mainstay of therapy for retinal conditions, also hold promise for corneal applications and may play a particularly auspicious role in graft survival after penetrating keratoplasty.2
A Better Way?
Conventional therapy for corneal neovascularization includes steroids, thought to suppress activation and migration of macrophages, mast cells, cytokines and other inflammatory cells promoting angiogenesis.2,6,7 Steroids are also often used in combination with oral matrix metalloproteinase (MMP) inhibitors such as doxycycline in an effort to regress abnormal corneal vasculature. These techniques are limited in efficacy, however, and lead to well-known side effects of topical steroids including cataracts and glaucoma. Nonsteroidal anti-inflammatory medications, photodynamic therapy, laser photocoagulation, fine needle diathermy and conjunctival, limbal and amniotic membrane transplantation have also been used with varying success.2,6
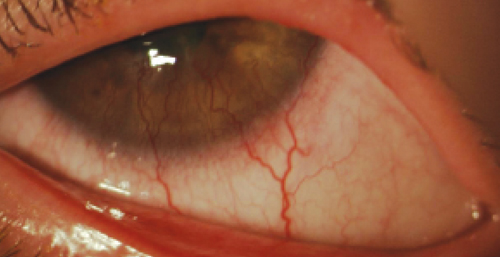 | |
| Vessel encroachment into the cornea in a case of neovascularization due to hypoxic stress. Might this be a patient who would benefit from anti-VEGF? |
Topical anti-VEGF therapy for corneal neovascularization has been investigated off-label using both bevacizumab and ranibizumab, which are monoclonal antibodies against VEGF and traditionally used for retinal indications.2,8,9 Both target VEGF-A, leading to inhibition of abnormal blood vessel formation and decreased vascular permeability. Bevacizumab has been used more often in off-label indications and in studies as a result of its increased affordability. Bevacizumab was originally approved for metastatic colorectal cancer, but has long been used in ophthalmology for off-label therapy of wet AMD, proliferative diabetic retinopathy and iris rubeosis.2,10
Therapy with anti-VEGF medications has been studied both in subconjunctival and topical use and has shown promise in the treatment of herpetic keratitis, recurrent pterygium, corneal transplant rejection and Stevens-Johnson syndrome.11 Multiple studies confirm the effectiveness of topical bevacizumab in reducing corneal neovascularization in experimental animal models, and human use has shown significant reductions in abnormal vasculature, even in patients recalcitrant to traditional anti-inflammatory therapies.2,6 Early treatment appears more efficacious in both animal and human models, with both topical and subconjunctival therapy.6,12,13 Chronic vascular conditions tend not to respond as well to therapy as active or acute angiogenesis.6,12
Clinically, corneal neovascularization can be seen after infectious, inflammatory and traumatic events.1,2 Inflammatory stress tips the balance of growth and inhibitory factors in favor of angiogenesis and leads to the growth of new, abnormal vasculature.4 The same can be seen under hypoxic conditions, such as contact lens overwear.2 The resulting neovascularization may be deep, stromal or may present as superficial vascular pannus, depending on the ocular insult.2,14 Deep and stromal neovascularization may be associated with interstitial and disciform changes seen in herpetic keratitis, while superficial changes are typically associated with ocular surface disease.2
Other bacterial, viral, protozoan and fungal antigens may also induce a keratitis that can lead to subsequent neovascularization. Trauma (including chemical burns), ischemia (i.e., limbal stem cell deficiency), and inflammatory conditions may also promote the abnormal vasculature.2,11,15 Autoimmune diseases (e.g., Stevens-Johnson syndrome, graft rejection and cicatricial pemphigoid) and corneal degenerations (e.g., pterygium and Terrien’s marginal degeneration) have further been implicated in corneal neovascularization.2,11,15 Perhaps the most widespread application, however, lies in corneal transplantation, where recipient neovascularization before transplantation doubles the risk of graft rejection, and where increases in graft survival after anti-VEGF therapy have been demonstrated in animal models.2
Growth Opportunity
Clearly, there is a role for anti-VEGF therapy in corneal neovascularization, and its potential anterior segment indications are plentiful. Areas for further research include determining the ideal administration, route, dosage and formulation, and whether a targeted combination therapy for multiple growth factors is necessary to completely regress vasculature in these patients. While large, randomized studies are required to firmly establish the safety and breadth of corneal indications, it seems clear that anti-VEGF therapy will have an increasingly significant role in the management of corneal neovascularization patients moving forward, and that corneal specialists will have a more efficacious tool at their disposal when treating this potentially blinding condition.
The authors would like to acknowledge Stephanie Fromstein, OD, for her invaluable contributions to this article.
1. Chang JH, Gabison EE, Kato T et al. Corneal neovascularization. Curr Opin Ophthamol. 2001; 12: 242-249.
2. Chang JH, Garg NG, Lunde E et al. Corneal neovascularization: an anti-VEGF therapy review. Surv Opthalmol. 2012 ; 57(5): 415-429.
3. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998; 43: 245-269.
4. Kvanta A. Ocular angiogenesis: the role of growth factors. Acta Opthalmol Scand. 2006.; 84: 282-288.
5. Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 I the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004; 82: 557-563.
6. Papathanassiou M, Theodoropoulou S, Analitis A et al. Vascular endothelial growth factor inhibitors for the treatment of corneal neovascularization: a meta-analysis. Cornea. 2013; 32(4): 435-444.
7. Phillips K, Arffa R, Cintron C et al. Effects of prednisolone and medroxyprogesterone on corneal wound healing, ulceration and neovascularization. Arch Opthalmol. 1983; 101: 640-643.
8. Avila MP, Farah ME, Santos A et al. Three-year safety and visual acuity results of epimacular 90strontium/90yttrium brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Retina. 2011; 32(1): 10-18.
9. Krebs I, Lie S, Stolba U et al. Efficacy of intravitreal bevacizumab (Avastin) therapy for early and advanced neovascular age-related macular degeneration. Acta Opthalmol. 2009; 87: 611-617.
10. Cheng SF, Dastjerdi MH, Okanobo A et al. Short-term topical bevacizumab in the treatment of stable corneal neovascularization. Am J Ophthalmol. 2012; 154: 940-948.
11. Ambati B. Corneal applications for anti-VEGF agents. Adv Oc Car. 2011: 24-25.
12. Papathanassiou M, Theodossiadis PG, Liarakos VS et al. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Opthalmol. 2008; 145: 424-431.
13. Stephenson M. Anti-VEGF for CNV: questions remain. Rev Ophthalmol. 2011. Published online
14. Ellenberg D, Azar DT, Hallak JA et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010; 29: 208-248.
15. Bock F, Konig Y, Kruse F et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 281-284.


