Corneal disease is the fourth leading cause of global blindness—after cataract, glaucoma and age-related macula degeneration—and affects more than 10 million people through a wide variety of infectious and inflammatory eye diseases that result in scarring and loss of best corrected vision.1 Corneal transplantation procedures, including standard full thickness penetrating keratoplasty, endothelial keratoplasty and deep anterior lamellar keratoplasty, are accepted treatments for many of these conditions and enjoy tremendous success.
However, alternative options are still needed to provide corneal visual rehabilitation to millions of patients in need. In this article, I will discuss what is currently available in the field of artificial corneas, also known as keratoprostheses, as well as exciting new research in the area of biosynthetic corneas. There have been many improvements in keratoprosthesis technologies and outcomes in the past few years, so be sure to consider these options in your armamentarium of treatment.
Growing Success

1. Alphacor keratoprosthesis.
Marc Muraine, M.D., and colleagues conducted a retrospective study over a decade and determined that the survival rates of corneal grafts was 79% after one year, 73% after two years, 59% after five years and 50% after 10 years.2 The greatest survival rate was for keratoconus at almost 95%, followed by herpetic keratitis, pseudophakic keratopathies and post-traumatic keratopathies. The lowest success rate of 11.1% occurred with re-grafts.2
Keryn Williams, M.D., reported better statistics with survival rates of 91% at one year, 72% at five years and 69% at seven years.3 The three greatest causes for graft failure were said to be rejection, infection and glaucoma.3
Francis Price, M.D., and Marianne Price, Ph.D., confirm the improving success rates for adult keratoplasty over the past quarter century, citing improvements in surgical techniques, procedures, devices, drugs and postoperative regimens.4
However, even in the best of situations, graft failure is a dreaded complication and potential reality for some patients. A report from the Collaborative Corneal Transplant Studies named the most influential factors for graft failure as young age, previous grafts, previous anterior segment surgery, preoperative glaucoma, anterior synechiae, stromal vessels and a primary diagnosis of chemical burn.5
Corneal Transplants

2. Alphacor keratoprosthesis.
Even with all its reported successes, only about 100,000 standard corneal transplants are performed worldwide yearly, of which approximately half are completed in the United States.6,7 The deficit of appropriate human donor corneas, as well as the lack of trained surgeons and eye banks in developing countries, contributes to this unmet global need.
These obstacles, coupled with the already described graft failure risks and challenges, add further complications; Jeffrey Ing, M.D., and colleagues found a 21% overall failure rate at 10 years.8 These rates further decline when repeat grafts are needed. In a study by Valery Berdudsky, M.D., and colleagues, the mean survival period for an initial regraft was 12.3 months, 11.4 months for a second and only 8.7 months for a third.9
Despite the advancements in modern surgical techniques and medications employed to treat patients with advanced corneal disease, there still remain certain groups of patients who continue to have a poor prognosis for success post-corneal transplantation, including those with autoimmune diseases such as Stevens-Johnson syndrome, mucous membrane pemphigoid, limbal stem cell insufficiency, chemical burn, congenital aniridia, herpetic keratitis and pediatric scarring.10,11
Iknur Tugal-Tutkun, M.D., Yonca Akova, M.D., and C. Stephen Foster, M.D., researchers from the Massachusetts Eye and Ear Infirmary in Boston, reported on a series of 16 eyes in 13 patients with advanced ocular cicatricial pemphigoid, Stevens-Johnson syndrome or toxic epidermal necrosis who underwent penetrating keratoplasty for corneal perforation or scarring. After a mean follow up period of 4.6 years, 50% had clear grafts, but only 18.7% had visual acuity of better than 20/200 due to persistent epithelial defect, stromal ulceration, perforation and rejection; this confirmed the poor prognosis for success in this subset of patients.12
For children, the need for swift vision rehabilitation is critical for amblyopia prevention. Unfortunately graft failure rates are higher in this population than in adults, with failure rates of 54% at one year for patients under 15 years old.13 This has been attributed to the more technically difficult surgery, corneal thinness and pliability, low scleral rigidity and smaller graft size. Additionally, the inflammatory response and risk of irregular astigmatism is greater in children than what is typically encountered in adults.14
So, it is clearly evident that alternative options are needed to provide corneal visual rehabilitation to millions of patients in need.
Artificial Cornea Options

3. Boston keratoprostheis.
The notion of artificial corneas is not new. Guillaume Pellier de Quengsy, M.D., first suggested the idea for keratoprostheses in 1789, but the first procedure didn’t take place until 1855.15
Keratoprostheses are typically made from a biologically inert material and have usually been reserved for those eyes for whom the chance of success with standard corneal transplantation was limited, such as severe autoimmune disease or chemical burns, or for patients who have already had multiple, failed re-grafts.
There are three main keratoprostheses used in clinical practice today: the Boston Keratoprosthesis (Boston KPro)Type I and Type II, Addition Technology Inc’s AlphaCor and the osteo-odonto keratoprosthesis (OOKP), first described by Bietti Strampelli, M.D., in 1965.16
• Boston KPro Type I and II: The Type I utilizes a donor cornea and leaves the eyelids intact, while the Type II is reserved for eyes with severe symblepharon and keratinization and is inserted through the eyelids. For the purposes of this article, only Type I will be discussed.
The Boston Type I consists of a polymethyl methacrylate (PMMA) optic and back plate with donor tissue clamped between the two. The assembled donor tissue and KPro is sutured into a trephined host, just as with a penetrating keratoplasty.17 Since its FDA clearance in 1992, it has undergone a number of improvements in both its design and therapeutic management.18,19 The more predictable surgical outcome and satisfactory visual experience has resulted in a significant uptick in the number of procedures performed. In 2009, more than 1,100 cases were performed—a 3,000% increase since 2002.20
Eight round holes in the back plate have reduced the risk of stromal melt and necrosis, as they provide the donor tissue easier access to essential nutrients within the aqueous. In a retrospective study of 157 eyes, half with holes in the backplate and half without, only 10% of eyes with the new design developed a corneal melt compared to 50% with the original design.21 Another design modification in 2003 was the addition of a titanium locking ring to secure the backplate to the frontplate, replacing the original threaded design and allowing for a more stable device. Bilal Khan, M.D., and colleagues also describe success with a titanium, rather than a PMMA backplate, which they attribute to its resiliency and thinner design.22
• AlphaCor: Previously known as the Chirila KPro, AlphaCor was the first keratoprosthesis to attempt to mimic a human cornea. It is a one piece “core and skirt” hydrogel implant, made from poly-2-hydroxyethyl methacrylate (PHEMA), which is permanently joined as an interpenetrating polymer network. This avoids the risk of epithelial downgrowth or aqueous leakage, both problems associated with the PMMA Boston KPro. It was first implanted in human eyes in 1998 in Australia and approved by the FDA in 2003.23,24
AlphaCor is designed to not rely on human donor tissue and to provide reversibility to standard keratoplasty if the need should arise. It is intended for use in patients with unilateral corneal disease who exhibit a reasonable tear film. It is contraindicated for those with severe ocular surface disease and possibly a history of ocular herpes simplex.
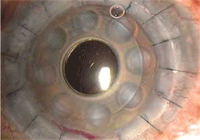
4. Boston keratoprostheis.
Implantation is a two-stage surgery, spaced approximately three months apart to allow for biointegration of the cells to “grow into” the host matrix by ingrowth of stromal fibroblasts, which allows for long-term anchoring. In the first stage, a keratectomy is performed and the device is implanted into the surgical bed. After this phase, patients can expect limited sight. During the second stage, a central region is excised from the patient’s central corneal flap, thus “opening the window” to the underlying implant.
The major complications associated with the AlphaCor appear to occur because of exposure of the plastic implant to the eyes’ exterior and the breakdown of the interface between the implant and the surrounding external ocular tissues, which is associated with this second stage of the procedure. These include stromal melting, fibrous reclosure of the posterior lamellar opening, deposits on the optic, chronic inflammation, retroprosthetic membranes, infection, poor biointegration and device extrusion.23-25
According to one study where AlphaCor results were retrospectively reported on 15 patients in the Czech Republic, the authors found the survivability rates of the implants to be 87% at one year, 58% at two years and 42% at three years.23 Postoperative complications included stromal melts in nine cases and post-implant membranes in three patients. Visual acuities ranged from count fingers to 20/125.26
Another study in Germany also noted corneal stromal melts in three patients, with some ultimately having to undergo explantation and replacement with a human graft.27 Vanessa Ngakeng, M.D., noted decreased melting by use of topical medroxyprogesterone (MPG) and by skipping the second step of the procedure, the removal of the central host cornea over the AlphaCor implant.27 By doing so, Ngakeng also found a reduction of deposits on the implant. While this did not improve the ultimate visual function of the implant, it was found to reduce pain and photophobia, which became the author’s criterion for surgical success.25 However, unlike penetrating keratoplasty, AlphaCor does not seem to exacerbate glaucoma and has an extremely low rate of endophthalmitis and retinal detachment.24
• Osteo-odonto-keratoprosthesis: OOKP, first described in 1965, utilizes the patient’s own tooth and alveolar bone to support a centrally mounted PMMA optical cylinder.16 OOKP has typically been used for patients with bilateral corneal blindness from severe ocular and systemic pathologies who are not good candidates for conventional penetrating keratoplasty.28 The OOKP has been shown to have success in patients with severe tear deficiency, cicatrization and chemical injury, likely due to the use of autologous tissue.29
Like the AlphaCor, OOKP implantation is a two-stage procedure. In the first phase, the eye is covered with buccal mucosal membrane graft. A tooth and its surrounding alveolar tissue from the patient is harvested, an osteo-odonto-acrylic lamina (OOAL) with a central opening is made and buried under the contralateral lower eyelid to promote vascularization and retard infection. Three months later, the OOAL is implanted at the anterior surface of the globe under the mucosal membrane graft.30
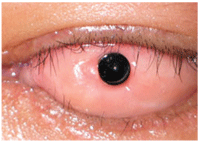
5. Osteo-odonto-keratoprosthesis (OOKP).
In a non-comparative retrospective case study of 36 consecutive patients over a 10-year period, the retention rate of the OOKP was 72%, with resorption of the OOKP as the primary factor for failure.31 Eighty-three percent of patients experienced vision improvement, with 25% reporting severe complications including endophthalmitis, retinal detachment and glaucoma.28 Giancarlo Falcinelli, M.D., reported that 85% of implants are intact at 18 years.32
Complications with Keratoprosthesis
There have been several reported obstacles associated with keratoprosthesis implantation, including the relative complexity of the surgery (as compared to penetrating keratoplasty), as well as the time and cost constraints. Complications from the surgery include formation of a retroprosthetic membrane, glaucoma, persistent epithelial defect, sterile vitritis, endophthalmitis, fungal keratitis and corneal necrosis.
In a survey of one physician’s experience over a four-year period, 50 eyes in 49 patients were implanted with the device. Preoperative vision in all cases was worse than 20/200. Postoperative vision was 20/100 or better in 67% of eyes at six months, 75% at one year, 69% at two years.33
The most common complication was the formation of retroprosthetic membranes in 22 eyes.33 Nd:YAG laser is a safe and effective technique for removal of these retroprosthetic membranes.34,35 Rebecca Stacy, M.D., and colleagues characterized dense membranes growing behind explanted Boston Type I keratoprosthesis from four patients that were resistant to YAG membranotomies. The material was found to originate in the patients from multiple sources including the patient’s original stroma—not epithelium—which grew downward behind the implant in vascularized sheets, the patients iris stroma and a retroiridial membrane from the metaplastic lens epithelium.36 The author recommended incorporating antifibroproliferative agents in the surgery to reduce membrane formation.
Glaucoma is a serious concern because the eyes that would benefit most from keratoprosthesis are generally also at a higher risk of developing glaucoma. Joseph Tauber, M.D., reported that 26% of 111 patients with ocular cicatricial pemphigoid had preoperative glaucoma; Peter Netland, M.D.,had an incidence of 36% preoperatively, with an additional 28% post-keratoprosthesis surgery—thus accounting for almost two-thirds of the 55 patients studied.37,38 However, 63% showed improved postoperative visual acuity and 81% improved with intraocular pressure control via drainage implants, so keratoprosthesis would still be considered a success.38
Future Research
Despite the successes, there are still limitations and complications associated with artificial plastic keratoprostheses and other alternatives are needed. The use of glass-reinforced hydroxyapatite (GRHA), already used in orthopedics and dentistry, shows some promise as a biocompatible keratoprosthesis.39 Researchers at Stanford University are also working on novel hydrogel materials for keratoprosthetics that will be both biocompatible and maintain structural integrity.40
Other ongoing research focuses on the development of biosynthetic corneas, designed to stimulate endogenous corneal regeneration; they will allow not only restoration of vision but eliminate risks of rejection, disease transmission and long-term immunosuppressive therapy, as well as promote corneal physiologic function. Laboratory manufactured corneas could also be potentially less expensive than human donor tissue.41,42
In a report of a two year follow up of 10 keratoconic patients who received biosynthetic corneas composed of cross-linked recombinant human collagen, all implants remained clear and free of infection or rejection.43 While visual acuity was inferior to those who had received human donor tissue, previously intolerable contact lenses were able to provide equivalent acuity.43 Stephanie Proulx, M.D., and colleagues have demonstrated the feasibility of producing tissue-engineered corneas using transformed fibroblasts, epithelial and endothelial cells, without needed exogenous biomaterial.44
Of the 10 million people worldwide who suffer from corneal blinding diseases, only a mere fraction will recover meaningful sight through traditional corneal surgery. The field of keratoprosthetic implantation has seen tremendous strides in the last decade and newer, more biocompatible materials along with recombinant human collagen are being investigated and show tremendous promise. Though certainly not a panacea for all, it could offer the prospect of improved vision for some previously hopeless cases.
Dr. Chaglasian is an associate professor at the Illinois College of Optometry in Chicago and an associate at Mack Eye Center in Hoffman Estates, Ill.
1. Witcher J, Srinivasan M, Upadhyay MP. Corneal Blindness: A global perspective. Bulletin of the World Health Organization. 2001;79(3):214-21.
2. Muraine M, Sanchez C, Watt L, et al. Long-terms results of penetrating keratoplasty. A 10-year plus retrospective study. Graefe’s Arch Clin Exp Ophthalmol. 2003;241(7):571-6.
3. Williams KA, Muehlberg SM, Lewis RF, Coster DJ. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye (Lond). 1995;9(Pt.2):219-27.
4. Price FW, Price MO. Adult keratoplasty: has the prognosis improved in the last 25 years? Int Ophthalmol. 2008 Jun;28(3):141-6.
5. Maguire MG, Starck WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994 Sep;101(9):1536-47.
6. Corneal Research Foundation of America. Available at:
www.cornea.org/index.php/research/corneal_transplant/artificial_cornea (Accessed July 2011).
7. Eye Bank Association of America. Available at:
www.restoresight.org/about-us/frequently-asked-questions/#45 (Accessed July 2011).
8. Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998 Oct;105(10):1855-65.
9. Bersudsky V, Blum-Hareuveni T, Rehany U, Rumelt S. The profile of repeated corneal transplantation. Ophthalmology. 2001 Mar;108(3):461-9.
10. Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal autograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002 Jun;109(6):1159-66.
11. Lee H, Khan R, O’Keefe M. Aniridia:current pathology and management. Acta Ophthalmol. 2008 Nov;86(7):708-15.
12. Tugal-Tutkun I, Akova YA, Foster CS. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology. 1995 Apr;102(4):576-85.
13. Huang C, O’Hara M, Mannis MJ. Primary pediatric keratoplasty: indications and outcomes. Cornea. 2009 Oct;28(9):1003-8.
14. Nallasamy S, Colby K. Keratoprosthesis: procedure of choice for corneal opacities in children? Semin Ophthalmol. 2010 Sep-Nov;25(5-6):244-8.
15. Chirila TV, Hicks CR. The origins of the artificial cornea. Pellier de Quengsy and his contribution to the modern concept of keratoprosthesis. Gesnerus. 1999;56(1-2):96-106.
16. Strampelli B, Valvo A, Tusa E. Osteo-odonto-keratoprosthesis in a case treated for anchylobepharon and total simbleraphon. Ann Ottalmol Clin Ocul. 1965 Jun;91(6):462-79.
17. Doane MG, Dohlman CH, Bearse G. Fabrication of a keratoprosthesis. Cornea. 1996 Mar;15(2):179-84.
18. Traish AS, Chodosh J. Expanding application of the Boston type I keratoprosthesis due to advances in design and improved post-operative therapeutic strategies. Semin Ophthalmol. 2010 Sep-Nov;25(5-6):239-43.
19. Klufas MA, Colby KA. The Boston Keratoprosthesis. Int Ophthalmol Clin. 2010 Summer;50(3):161-75.
20. Ciolino JB, Dohlman CH. Biologic keratoprosthesis materials. Int Ophthalmol Clin. 2009 Winter;49(1):1-9.
21. Harisi-Dagher M, Khan BF, Schaumberg DA, Dohlman CH. Importance of nutrition to corneal grafts when used as a carrier of the Boston keratoprosthesis. Cornea. 2007 Jun;26(5):564-8.
22. Khan BF, Harissi-Dagher M, Khan DM, Dohlman CH. Advances in Boston Keratoprosthesis: enhancing retention nd prevention of infection and inflammation. Int Ophthalmol Clin. 2007 Spring;47(2):61-71
23. Hicks CR, Crawford GJ, Tan DT et al. AlphaCor cases: comparative outcomes. Cornea 2003 Oct;22(7):583-90.
24. Hicks CR, Crawford GJ, Dart JK, Grabner G.K, et al. AlphaCor: clinical outcomes. Cornea. 2006 Oct;25(9):1034-42.
25. Ngakeng V, Hauck MJ, Price MO, Price FW Jr. AlphaCor keratoprosthesis: a novel approach to minimize the risks of long-term postoperative complications. Cornea. 2008 Sep;27(8):905-10.
26. Jirásková N, Rozsival P, Burova M, Kalfertova M. AlphaCor artificial cornea: clinical outcome. Eye (Lond). 2011 Jun 17. [Epub ahead of print]
27. Holak SA, Holak HM, Bleckmann H. AlphaCor keratoprosthesis: postoperative development of six patients. Graefes Arch Clin Exp Ophthalmol. 2009 Apr;247(4):535-9.
28. Hille K, Grabner G, Liu C, et al. Standards for modified osteoodontokeratoprosthesis (OOKP) surgery according to Strampelli and Falcinelli: the Rome-Vienna Protocol. Cornea. 2005 Nov;24(8):895-908.
29. Zerbe BL, Belin MW, Ciolino JB; Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006 Oct;113(10):1779.e1-7.
30. Liu C, Okera S, Tandon R, et al. Visual rehabilitation in end-stage ocular surface disease with the osteo-odonto-keratoprosthesis; results from the UK. Br J Ophthalmol. 2008 Sep;92(9): 1211-7.
31. Liu C, Hille K, Tan D, et al. Keratoprosthesis surgery. Dev Ophthalmol. 2008;41:171-86.
32. Falcinelli GC, Falsini B, Taloni M, et al. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness; long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005 Oct;123(10):1319-29.
33. Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009 Apr;116(4):640-51.
34. Chak G, Aquavella JV. A safe Nd:Yag retroprosthetic membrane removal technique for keratoprosthesis. Cornea. 2010 Oct;29(10):1169-72.
35. Bath PE, McCord RC, Cox KC. Nd:YAG laser discussion of retroprosthetic membrane: a preliminary report. Cornea. 1983;2:225-8.
36. Stacy RC, Jakobiec FA, Michaud NA, et al. Characterization of retrokeratoprosthetic membranes in the Boston type 1 keratoprosthesis. Arch Ophthalmol. 2011 Mar;129(3):310-6.
37. Tauber J, Melamed S, Foster CS. Glaucoma in patients with ocular cicatricial pemphigoid. Ophthalmology. 1989;96(1):33-7.
38. Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998 Apr;105(4):751-7.
39. Santos L, Ferraz MP, Shirosaki Y, et al. Degradation studies and biological behavior on an artificial cornea material. Invest Ophthalmol Vis Sci. 2011 Jun 16;52(7):4274-81.
40. Hartmann L, Watanabe K, Zheng LL, et al. Toward the development of an artificial cornea: Improved stability of interpenetrating polymer networks. Biomed Mater Res B Appl Biomater. 2011 Jul;98(1):8-17.
41. Liu W, Merrett K, Griffith M, et al. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials. 2008 Mar;29(9):1147-58.
42. Lagali N, Fagerholm P, Griffith M. Biosynthetic corneas: prospects for supplementing the human donor cornea supply. Expert Rev Med Devices. 2011 Mar;8(2):127-30.
43. Fagerholm P, Lagali NS, Merrett K, et al., A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010 Aug 25;2(46):46ra61.
44. Proulx S, d’Arc Uwamaliya J, Carrier P, et al. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol Vis. 2010 Oct 29;16:2192-201.


