 |
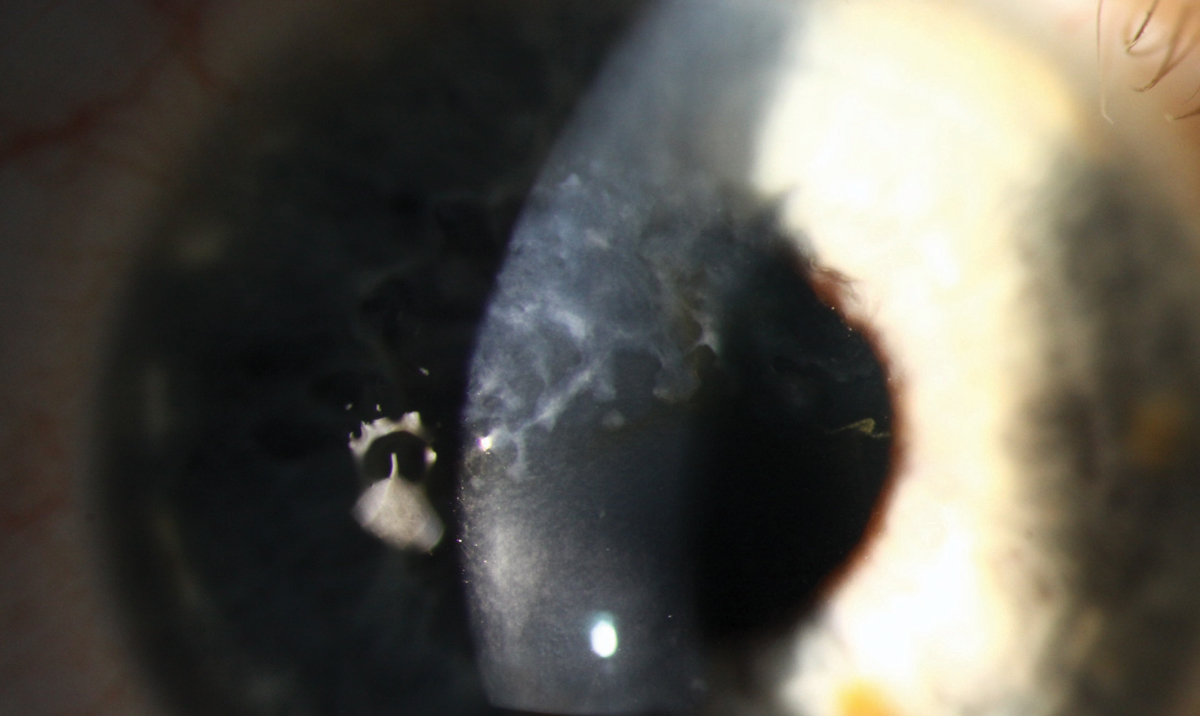
This 50-year-old male with keratoconus underwent collagen crosslinking in 2013 and subsequently developed white, elevated subepithelial deposits within the visual axis, requiring superficial keratectomy (SK). He presented in 2016 for a scleral lens evaluation secondary to persistent foreign body sensation. At the time, best-corrected vision was 20/25; however, in 2018 it had dropped to 20/70. An increased density of central scarring was noted and OCT showed deposits localized to the subepithelial region. SK is again indicated.
Amyloid is an insoluble, abnormal protein that aggregates in the cornea, numerous other ocular structures and elsewhere in the body.1 Amyloidosis is an inherited disorder caused by defects of the TGFBI gene. Depending on the type and position of the amino substitution, aggregates may be fibrillary or amorphously globular. The protein composition explains the different corneal phenotypes and depth of deposition. It typically appears as a greyish-white deposit.
Amyloidosis can be primary or secondary, with each further divided into systemic or localized forms. Primary corneal amyloidosis includes autosomal-dominant entities such as lattice, granular and Avellino dystrophies, as well as autosomal recessive drop-like gelatinous dystrophy.
Secondary localized amyloidosis, while uncommon, has been reported following corneal trauma and wound healing. It has also been associated with trichiasis, keratoconus and numerous ocular inflammatory and degenerative conditions.2
While the mechanism of secondary localized corneal amyloidosis is unknown, research suggests it results from altered transforming growth factor β–induced protein, produced by injured keratocytes. Lactoferrin in the tear film and keratoepithelin from the corneal epithelium may also be involved.2
While this patient clinically appears to have secondary amyloidosis, his specimen will be sent to the pathology lab after his next SK.
 |
1. Araki-Sasaki K1, Hirano K, Osakabe Y, et al. Classification of secondary corneal amyloidosis and involvement of lactoferrin. Ophthalmology. 2013;120(6):1166-72. |


