In every practice, there are hundreds—perhaps even thousands—of patients who suffer from some form of ocular dryness. Many monikers have been assigned to this condition (ocular surface disease, keratoconjunctivitis sicca, dysfunctional tear syndrome, etc.), but regardless of the terminology, individuals who struggle with these maladies represent a tremendous opportunity for us to provide symptomatic relief and ongoing care. They are also a potential source of practice growth. Many of these individuals can be successfully treated with one or more of the conventional treatment modalities that are in common use today.
But unfortunately, a small subset of these patients suffers from severe, potentially sight-threatening dryness. They may report minimal or no relief from artificial tears, punctal plugs and other commonly prescribed therapies.
In this article, we will review the current thinking on the various pathogeneses of dry eye and methods to manage and treat the more severe forms.
What the Experts Say
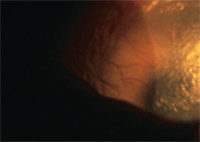
This patient has corneal neovascularization and extreme ocular dryness secondary to Stevens-Johnson syndrome.
During the past two decades, several panels of experts in the field of dry eye and ocular surface disease have pooled their collective expertise to reach some consensus on the causes of dry eye and devise paradigms for treating these conditions. The National Eye Institute/Industry Workshop on Clinical Trials in Dry Eyes, the Dry Eye WorkShop (DEWS) and the Delphi Panel helped us to better understand the underlying pathophysiologies of dry eye and established logical paradigms for treatment.1-4 One important concept that came out of these panels is that dry eye can be caused by inadequate lacrimal layer production (aqueous tear deficiency) or excessive evaporation of the tear film (evaporative dry eye), which is often associated with meibomian gland dysfunction.
The Delphi Panel expanded on the initial classification of aqueous tear deficiency vs. evaporative dry eye, because in many cases, dry eye sufferers may have both entities, and because earlier classification schemes did not emphasize the contribution of inflammation to the pathogenesis of dry eye.4 You may think that arriving at a definition for “severe dry eye” is simple, but when you consider the numerous studies and conferences on dry eye, there is currently no common consensus on a universal description of the more severe form of this condition.
What is Severe Dry Eye?
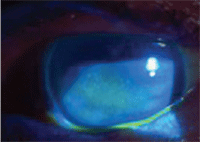
This 87-year-old female with
Sjögren’s syndrome shows significant corneal staining with fluorescein.
Her presenting complaints were ocular pain and blurred vision.
The DEWS report assimilated information from the NEI workshop and the Delphi panel to formulate criteria for higher levels of severity. These include severe and frequent discomfort; chronic, constant or disabling visual symptoms; some degree of conjunctival injection; marked central corneal staining with possible erosions; filamentary keratitis with mucin clumping and increased tear debris; advanced meibomian gland changes that may include trichiasis and keratinization; and a Schirmer test score of 2mm to 5mm.2
Severe dry eye may result from extreme dysfunction of the lacrimal glands, the meibomian glands or both. In 1930, Henrik Sjögren first published a description of five patients with marked dryness of the eyes and mouth. In his doctoral dissertation, he referred to the condition as “keratitis sicca.”5 Histological evaluation subsequently revealed that Sjögren’s syndrome (SS) results from chronic autoimmune disease involving proliferation of T lymphocytes and B lymphocytes in exocrine glandular cells. Sjögren’s syndrome may occur independently (primary Sjögren’s) or in conjunction with other autoimmune disease (secondary Sjögren’s). The expression of cytokines, including tumor necrosis factor- (TNF- ), causes destruction of the acini of secretory cells.6 We now know that both forms of SS may cause severe dry eye.
One study evaluated the effectiveness of punctal occlusion for treating severe SS. Researchers used one eye as a control and performed punctual occlusion in the other eye. They found that occlusion improved comfort and rose bengal scores in the treated eye, but had no effect on Schirmer scores and mucus debris scores.7
The lack of effect on the Schirmer test in SS has been reported previously and may be due to the deficiency of reflex tearing found in this patient group.8
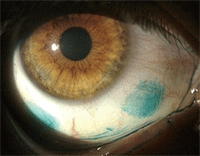
This contact lens wearer has severe dry eye that is evidenced by marked lissamine green staining of the conjunctiva.
Inflammation has become a well-recognized factor in the pathogenesis of non-SS ocular dryness; Restasis (Cyclosporin A 0.05%, Allergan) is a widely prescribed therapeutic agent approved for instillation every 12 hours. The original Phase II and III trials did not demonstrate a statistically significant difference between the currently approved protocol and higher dosing frequency or concentration.9,10 One study evaluated the effect of more frequent dosing in individuals whose severe dry eye was refractory to twice-daily dosing of Cyclosporin A 0.05% after a period of at least four months. All these subjects’ severe dry eye was secondary to graft-versus-host disease or SS. Findings demonstrated that increasing dosing to t.i.d. or q.i.d. resulted in significant improvement in both the subjective complaints and the rose bengal score, but did not improve Schirmer values and the tear mucus scores.11 This study suggests that increasing the dosing or concentration of cyclosporine might benefit our patients with advanced dry eye.
Trachoma is another form of severe dry eye and is also a common cause of vision loss and blindness worldwide.12 Conjunctival cicatrization destroys the basic secretory system and obliterates the excretory ductules of the lacrimal gland, often resulting in severe ocular surface disease. Tissue biopsies that were taken from patients with active trachoma revealed infiltrative lymphoid follicles in the underlying conjunctival stroma. Specimens obtained from eyes with inactive disease showed subepithelial fibrous membranes, squamous metaplasia and loss of goblet cells. Acini and ducts of accessory lacrimal glands were damaged by subepithelial infiltration and scarring.12 Cyclosporine has been evaluated for treatment of severe trachomatous dry eye. After six months of therapy, researchers found statistically significant improvement in symptom scores and Ocular Surface Disease Index (OSDI) scores, rose bengal and fluorescein staining scores, tear film break-up time (TFBUT) values and Schirmer test values. Impression cytology also showed improvement of goblet cell density in 23 patients (71.88%).13
Severe dry eye may also occur as a result of chemical trauma, radiation or immunologic diseases that target mucous membranes.18-20 Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe acute adverse drug reactions that primarily affect the skin, but they may also involve ocular, oral and genital mucous membranes. Although similar in most respects, SJS typically affects less that 10% of body surfaces, whereas TEN affects greater than 30% of body surface.20 These conditions, as well as ocular cicatricial pemphigoid (OCP), may severely damage the ocular surface—particularly the mucous membranes. The most commonly reported complication of these conditions is severe dry eye.18-20
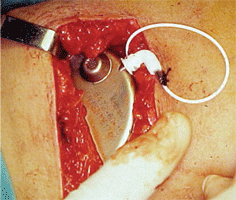
The pump is implanted in the abdominal
subcutaneous pocket.
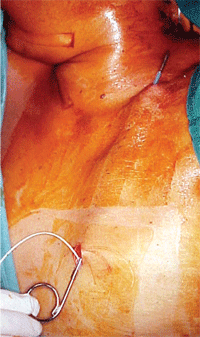
Through this
subcutaneous catheter the pump delivers fluid from the abdomen to the
upper conjunctival fornix. Courtesy: Juan Murube, M.D., Ph.D.
Treatment Opportunities
In the past decade, several excellent artificial tear products have been introduced. But, despite these advances, currently available artificial tear preparations do not include endogenous tear components such as epidermal growth factor, hepatocyte growth factor, fibronectin, neurotrophic growth factor, cytokines, mucins and vitamin A. These molecules play an important role in the maintenance of a healthy ocular surface.14 Several studies have compared use of autologous serum (AS) modified for use as artificial tears to conventional or non-preserved artificial tears.14,15 For example, after two weeks, patients assigned to AS eye drops (vs. subjects assigned to preservative-free artificial tears) showed improvement in mean TFBUT as well as fluorescein and rose bengal staining scores.15 Subjective symptom scores also showed significant improvement after two weeks of treatment.15
The process for preparing AS to artificial tears requires several steps. The blood is permitted to clot at 4º Celsius for 10 to 12 hours, and it is then centrifuged at 4,500rpm for 15 minutes to eliminate erythrocytes and leukocytes.16 The serum is separated in a laminar flow hood and diluted with sterile saline (0.9%) to a 20% concentration. Dilution is necessary to reduce levels of transforming growth factor (TGF-beta), which has been identified as an inhibitor of proliferation.16,17 Because AS is unpreserved, there is always the risk for contamination. Of 134 samples of AS prescribed for individuals after ocular surface surgery that were cultured in one study, only 13 were positive for contaminants. Nine of these were Staphylococcus epidermidis, and one was Staphylococcus aureus. But, none of the subjects had clinical or laboratory evidence of infection.17
In a study of severe conditions, such as SJS or TEN, causative agents included antibiotics (27.4%), antiepileptic drugs (26.5%), allopurinol (17%) and phenytoin (10%). Ten (8.5%) of these subjects died during the acute phase of disease, 69% had ocular involvement, and severe dry eyes and trichiasis were the two most common late complications.21 During the acute phase of the systemic disease, 32 subjects (82%) underwent aggressive treatment with systemic steroids equivalent to oral prednisolone 1mg/kg body weight per day.21
Topical ophthalmic steroids, antibiotics and lubricants were also prescribed for these patients, but in many cases, the outcome was severe dry eyes present in 46% of those with long-term follow-up care. Use of topical antibiotics during the acute phase of the disease was associated with late ocular complications, especially severe dry eyes. The authors recommended withholding use of antibiotics unless there is clear evidence of active infection.21
In very severe dry eye, surgical management may be necessary to prevent vision loss. In 1986, the Spanish ophthalmologist Juan Murube del Castillo, M.D., Ph.D., first reported transplantation of autologous submandibular gland (SMG). The gland is positioned in the temporal fossa, the vascular supply was anastomosed to the superficial temporal artery and vein, and the excretory duct was implanted into the upper conjunctival fornix.22 This was later performed in 15 eyes with cicatrizing conjunctivitis.23 Researchers reported significant improvement in subjective findings, such as reduced instillation of lubricants and objective parameters, including reduced signs of ocular surface damage. Unfortunately, the quality of secretions is salivary, and the osmolarity is lower than that of lacrimal secretions. In six of the 14 patients, this resulted in corneal edema and vision reduction secondary to chronic hypoosmolar condtions.23 Less than half of these subjects expressed a desire to have the procedure performed in the fellow. The authors recommended that autologous SMG transplantation be limited to individuals with Schirmer values less than 1mm.23
Individuals with xeropthalmia (possessing virtually no tear film), are among the most symptomatic and difficult to treat sufferers of extreme dry eye. Drs. Murube and Geerling described the use of an implantable pump reservoir in 21 patients with severe dry eye, whose Schirmer values were less than 2mm in five minutes.24 These subjects reported that they instilled artificial tears to the ocular surface every five to 10 minutes.24
Here’s how it’s done. The procedure is performed under general anesthesia. The skin is incised 2mm to 4mm below the ribcage, and the wound is deepened through the subcutaneous tissue.24 A pocket is formed over the underlying external oblique and straight up along the abdominal muscles. The pump/reservoir (Medtronic model IP 20.1, Anschütz & Co. GmbH) is implanted in the space. Two sequential subepithelial catheters connect the reservoir to the lateral aspect of the orbit and thereby the superior fornix. The device delivers a continuous flow of isotonic electrolyte solution 1ul/min with a total of approximately 1.5ml delivered to the surface of the eye each day. The reservoir must be refilled approximately every 40 days; this procedure is performed in the operating room under sterile conditions. Initial results of this procedure have been encouraging, and complications have been rare and not severe.24
Scleral lenses have been used to treat individuals with corneal scarring and ocular surface irregularities for decades, but edema secondary to hypoxia presented a challenge to practitioners using polymethyl methacrylate material. In 1990, there were 15 cases in which gas-permeable materials were successfully used to fabricate scleral lenses for individuals with a variety of conditions that caused reduced vision. Several of these subjects experienced significant improvement in vision and function.25
Perry Rosenthal, M.D., and Deborah S. Jacobs, M.D., reported fitting 33 consecutive patients with severe dry eye who were unresponsive to conventional therapy with the Boston Scleral Lens Prosthetic Device (BSLPD). All subjects’ disease was caused by chronic graft-versus-host disease secondary to allogeneic bone marrow or hematopoietic stem cell transplantation. The BSLPD is a custom-designed, gas-permeable contact lens that vaults the cornea and limbus and rests solely on the sclera. The tear layer trapped between the posterior surface of the lens and the cornea maintains a wet, oxygenated surface and protects the cornea from shear forces generated by blinking. The vast majority (97%) reported reduction in eye pain in the worse eye, and 73% of subjects reported a great improvement in quality of life. Sixty-one percent reported an outstanding improvement in reading. Properly fitted scleral lenses are another potential means of improving the vision and quality of life in individuals with severe dry eye.26,27
Improve Quality of Life Through Severe Dry Eye Management
Our initial diagnosis
was severe dry eye with secondary filamentary keratitis, which was
possibly exacerbated by the BAK-preserved product. Our treatment plan
included the following: • Debridement of the filaments with a sterile Kimura spatula.
Two days later, Margaret
described her pain and discomfort as “100% better, and the
non-preserved drops did not burn.” But, she reported irritation in the
right eye. After removal of the lens, instillation of fluorescein
showed staining. We recommended continuation of the bandage contact
lens O.S., and performed temporary occlusion of the upper and lower
puncta O.D. One week later, she continued to have no ocular discomfort
and reported that the temporary occlusion had enhanced comfort in the
right eye. We inserted a medium Parasol punctal occluder (Odyssey
Medical) in the lower puncta O.D. and continued use of the bandage lens
O.S. Although Margaret
continued to have vision problems for the remainder of her life, our
management of her severe dry eye enhanced her quality of life. Her case
demonstrates the importance of taking the time to investigate
complaints suggestive of dry eye, and if it is found to be causative
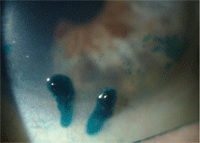
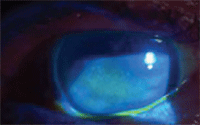
for the symptoms, managing dry eye appropriately. Two large mucin-coated filaments in this patient with severe dry eye. On initial presentation, Margaret’s left eye showed diffuse central corneal staining with sodium fluorescein.
“Margaret,” an 84-year-old female presented for evaluation
complaining of severe pain that was worse in the left eye. She had seen
numerous eye care providers for this condition, but none had
successfully ameliorated her symptoms. Margaret’s ocular health history
was positive for cataract surgery and laser treatment for exudative
macular degeneration. She had self-treated the long-standing discomfort
with a BAK-preserved over-the-counter artificial tear product. She
could count fingers at 3.00 feet O.S., and her best-corrected visual
acuity was 20/160 O.S. Biomicroscopy revealed severe tear film
reduction, grade 3+ corneal staining with fluorescein O.U. and
filamentary keratitis O.S.
• Bandage soft contact lens.
• Non-preserved artificial tears O.U., every two hours.
• Return to clinic in two days.
Know Your Options
Severe dry eye is a rare but bothersome condition that negatively affects both visual and general quality of life.28,29 It is encouraging to see the emergence of new treatments and technology that effectively reduce the untoward effects of severe dry eye on individuals who suffer from this debilitating condition. Given the potential for improving quality of life through these measures, it is incumbent upon eye care practitioners to provide or arrange for treatment of this disease. In order to do this, we must stay abreast of the many novel and effective new therapies and devices for the management of various forms of dry eye that may be introduced in the future.
1. Lemp MA. The Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995 Oct;21(4):221-32.
2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92.
3. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):93-107.
4. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006 Sep;25(8):900-7.
5. Rehman HU. Sjögren’s Syndrome. Yonsei Med J. 2003 Dec;44(6):947-954.
6. Meijer JM, Pijpe J, Bootsma H, et al. The future of biologic agents in the treatment of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2007 Jun;32(3):292-7.
7. Mansour K, Leonhardt CJ, Kalk WW, et al. Lacrimal punctum occlusion in the treatment of severe keratoconjunctivitis Sicca caused by Sjögren syndrome: a uniocular evaluation. Cornea. 2007 Feb;26(2):147-50.
8. Tuberville AW, Frederick WR, Wood TO. Punctal occlusion in tear deficiency syndromes. Ophthalmology. 1982 Oct;89(10):1170-2.
9. Stevenson D, Tauber J, Reis BL. Efficacy and safety of Cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000 May;107(5):967-74.
10. Sall K, Stevenson OD, Mundorf TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000 Apr;107(4):631-9.
11. Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea 2009 Oct 5. [Epub ahead of print].
12. Guzey M, Ozardali I, Basar E, et al. A survey of trachoma: the histopathology and the mechanism of progressive cicatrization of eyelid tissues. Ophthalmologica 2000;214(4): 277-84.
13. Guzey M, Karaman SK, Satici A, et al. Efficacy of topical cyclosporine A in the treatment of severe trachomatous dry eye. Clin Experiment Ophthalmol. 2009 Aug;37(6):541-9.
14. Schornack MM, Baratz KH. Ocular cicatricial pemphigoid: The Role of Scleral Lenses in Disease Management. Cornea. 2009 Oct 5. [Epub ahead of print]
15. Ghasemi H, Ghazanfari T, Ghassemi-Broumand M, et al. Long-term ocular consequences of sulfur mustard in seriously eye-injured war veterans. Cutan Ocul Toxicol. 2009;28(2):71-7.
16. Gueudry J, Roujeau JC, Binaghi M, et al. Risk Factors for the development of ocular complications of Stevens-Johnson Syndrome and toxic epidermal necrolysis. Arch Dermatol. 2009 Feb;145(2):157-62.
17. Kojima T, Higuchi A, Goto E, et al. Autologous serum eye drops for the treatment of dry eye diseases. Cornea 2008 Sep;27 Suppl 1:S25-30.
18. Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eye drops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005 Sep;140(3):565.
19. Quinto GG, Campos M, Behrens A. Autologous serum for ocular surface diseases. Arq Bras Oftalmol. 2008 Nov-Dec;71(6 Suppl):47-54.
20. Lagnado R, King AJ, Donald F, Dua HS. A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders. Br J Ophthalmol 2004 Apr;88(4):464-5.
21. Yip LW, Thong BY, Lim J, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy. 2007 May;62(5):527-31
22. Murube del Castillo J. Transplantation of salivary gland to the lacrimal basin. Scand J Rheumatol Suppl. 1986;61:264-267.
23. Geerling G, Collin JR, Dart JK. Ophthalmic experience with submandibular gland transplantation for severe dry eyes. Laryngoscope. 2009 Jul;119(7):1445-6.
24. Murube J, Geerling G. Mechanical Pump Dacryoreservoirs. Dev Ophthalmol. Dev Ophthalmol. 2008;41:269-82.
25. Schein OD, Rosenthal P, Ducharme C. A gas-permeable scleral contact lens for visual rehabilitation. Am J Ophthalmol. 1990 Mar 15;109(3):318-22.
26. Jacobs DS, Rosenthal P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. 2007 Dec;26(10):1195-9.
27. Segal O, Barkana Y, Hourovitz D, et al. Scleral contact lenses may help where other modalities fail. Cornea. 2003 May;22(4):308-10.
28. Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007 Mar;143(3):409-15.
29. Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005 Jan;46(1):46-50.
Dr. Townsend is in private practice in Canyon, Tex., and is an adjunct professor at the University of Houston College of Optometry. He is a member of the executive board of the Ocular Surface Society of Optometry (OSSO), a non-profit organization whose mission is to increase awareness and advance the understanding and management of dry eye and ocular surface disease. Information for joining OSSO can be found at [email protected].


