The visual consequences of keratoconus—which has historically been defined as a noninflammatory condition with hallmark progressive corneal thinning and steepening.—can be devastating for many patients.1 Corneal ectatic disorders often result in decreased acuity, increased ocular aberrations and, in some cases, the need for surgical intervention.1
Types of Ectasia
Because ectasia describes a physiological finding rather than a particular disease process, it is used in the context of various clinical entities that can arise by mechanical, degenerative or genetic factors.
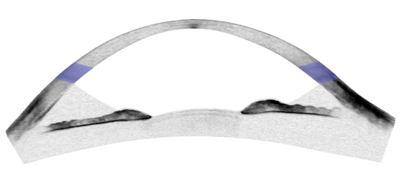 | |
| Anterior chamber segment optical coherence tomography image of thin, ectatic cornea in post-LASIK ectasia. | |
 | |
| Central 9mm segment optical coherence tomography image of thin, protruding cornea in keratoglobus. The central caliper measure of corneal thickness is 145µm. |
• Post-Surgical Ectasia. Iatrogenic ectasia can occur post-laser-assisted in situ keratomileusis (LASIK) or after a photorefractive keratectomy (PRK) procedure.1-2 Additionally, small-incision-lenticule extraction (SMILE) is a relatively novel refractive procedure that uses a femtosecond laser to remove a disc-shaped portion of stroma with no flap lift, achieving similarly successful results to those of LASIK. In two recent isolated case reports, ectasia following this procedure was documented.11,12
Common clinical findings in patients with post-refractive ectasia include a thicker resulting flap or thinner residual stromal bed than expected. However, post-refractive surgery ectasia can still occur in the absence of these findings.1-6 Overall, the prevalence of post-refractive surgery ectasia is estimated to be between 0.2% and 0.66%.1-6
Forme fruste (i.e., subclinical) keratoconus is an often-undiagnosed latent form of biomechanical instability in the cornea, which can be induced by refractive surgery and result in post-refractive surgery ectasia.6,8-9 Other risk factors for iatrogenic corneal ectasia include thin baseline cornea, irregular corneal thickness, high myopia (due to the need for an increased amount of ablated tissue) and young age at the time of refractive surgery.10 In all cases, unilateral presentation of keratoconus contraindicates surgery for the other eye because keratoconus is currently understood to be a bilateral disease.1-4
• Keratoconus. Considered to be the most common form of ectatic disorder, primary keratoconus affects at least one out of every 2,000 members of the general population.1-3 Usually, keratoconus presents in the second or third decade of life.1 Mandatory clinical findings to diagnose keratoconus include abnormal posterior ectasia, in addition to corneal thinning and abnormal corneal thickness distribution.1-2 According to the Global Consensus on Keratoconus and Ectatic Diseases, the pathophysiology of the condition likely includes genetic, biochemical, biomechanical and environmental components, but has no primary pathologic explanation.2 Induced ectasia may be unilateral or bilateral and secondary to mechanical processes in a predisposed cornea.2
• Pellucid Marginal Degeneration. Considered the second most common form of ectatic disorders, pellucid marginal degeneration presents between the third and fifth decade of life.1 It is characterized by inferior peripheral corneal thinning and a ‘bow-tie’ topographical map appearance of the cornea.
• Keratoglobus. A rare corneal thinning disorder that is characterized by general thinning and protrusion of the cornea. Keratoglobus may be present early in life, or may be acquired.1 Like primary keratoconus, the pathophysiologic and genetic etiology of pellucid marginal degeneration and keratoglobus remains unclear.
Today, the majority of patients with keratoconus or other forms of corneal ectasia are managed with specialty contact lenses, such as corneal or scleral rigid gas permeable lenses, rather than surgery. As such, optometrists trained in medical contact lenses are often the first line of care for this affected population. Being knowledgeable about the causes of these conditions can help to enable better clinical decisions and increase the ease of delivering patient education.
Biochemical Influences
Our knowledge about the causes of ectasia has come a long way since the condition’s discovery, largely because of advances in the way we measure the structure and composition of the cornea. Essentially, corneal thinning in keratoconus occurs when corneal collagen degrades.1,3 It has been suggested that this degradation could be related to alterations in enzyme activity from normal levels; for example, matrix metalloproteinase (MMP) levels have been shown to be elevated in keratoconic corneas, demonstrating a possible role for these enzymes in collagen degradation.3,12-14 Increased expression of MMPs is widely observed in human tissue in which inflammation is present.15 Elevated levels of other inflammatory mediators like interleukin-6 and tumor necrosis factor-α have also been found in the tears of keratoconus patients and an imbalance between pro-inflammatory and anti-inflammatory cytokinesas well.1,16 Aquaporins are cell membrane proteins that act as critical water channels for cells, and have been reported to be deficient in the epithelium of keratoconic corneas.38
Genetics
Despite decades of research on the possible genetic causes of keratoconus, its pathogenesis and association with other diseases remains poorly understood.3,18
Support for the potential of genetics or the environment playing a role in the progression of keratoconus has been introduced by a series of twin studies describing cases in which both siblings are affected.3,19 However, not all twin studies demonstrate evidence of both individuals developing keratoconus.3,20 Additionally, some linkage studies report genetic susceptibility related to gene mutation, while others have found no link between keratoconus and mutated genes.18,22-24
It does appear, however, that a positive family history for keratoconus is a risk factor for development of the disease, indicating a possible familial inheritance.18 Studies have estimated relatives of those with keratoconus to have a risk of 15 to 67 times greater than those with no family history of keratoconus.25 A recent supported hypothesis also suggests that consanguinity is a significant risk factor for keratoconus.31 However, to the best of our current knowledge, there are no direct genetic causative factors identified yet.
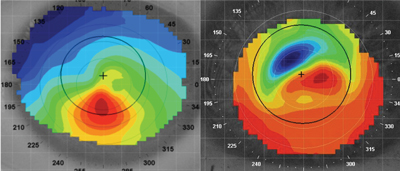 | |
| Left: Topography pattern showing inferior corneal steepening in keratoconus. Right: Topography pattern showing irregular astigmatism over the visual axis in post-LASIK ectasia. |
Interestingly, keratoconus can develop in the absence of certain conditions or in the presence of others. The medical literature documents associations between the disorder and Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome and Leber congenital amaneurosis.1-3,17-18 These correlations could be postulated to relate to the eye rubbing behavior or connective tissue deficiencies that commonly accompany many of these conditions.17
Thinning of other connective tissues of the body is not typically seen in keratoconus patients, however; the landmark Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study indicates no association with connective tissue disease.32
Oxidation
It is known that the cornea metabolizes oxygen to support its physiologic processes.17 Keratoconic corneas have been found to exhibit a decrease in levels of antioxidant enzymes such as glutathione, which are involved in the breakdown and elimination of reactive oxygen species.17,26-27 Relevant to keratoconus progression, several factors discussed in the literature that may lead to oxidative damage of corneal tissue—specifically, the stroma and Bowman’s membrane—are atopy, mechanical trauma and exposure to ultraviolet radiation.28
• Atopic Disease and Ocular Allergy. Atopy is defined as the genetic predisposition to develop allergic diseases like allergic rhinitis, asthma and atopic dermatitis (i.e., eczema). Atopy is typically associated with heightened immune responses to common allergens, especially those that are inhaled or ingested. An allergy is defined as a chronic condition that involves an abnormal reaction to an ordinarily harmless substance that functions as an allergen for that individual. In an allergic reaction, the immune system views an allergen as an invader and directs white blood cells to produce IgE antibodies, which attach themselves to mast cells to trigger a release of potent chemicals like histamine.29
Research suggests prevalence of keratoconus is associated with atopic disease and ocular allergy.1-3 Though there is evidence to suggest atopy may contribute to keratoconus, the association is most likely due to the tendency of patients with atopy and/or allergy to chronically rub their eyes to relieve irritation.2,30 As such, from a treatment standpoint, patients with coexistent keratoconus and atopic disease should be managed cooperatively by an eye care provider and immunologist.
• Mechanical Insult. The CLEK Study, which evaluated the connection between contact lens fitting techniques, apical changes and scarring in keratoconus, suggested that contact lens wear may be a risk factor for increased corneal scarring, implying that inadequate lens fit can negatively affect visual funcion.32 Equally important was the finding that because of the adverse effects poor vision can have on daily functionality, keratoconus patients who wear contact lenses (i.e., mostly rigid gas permeable lenses) generally have better scores on mental health and functionality measures than keratoconus patients who do not wear contact lenses.32
As noted above, eye rubbing can trigger unilateral or bilateral induced keratoconus, a cause-and-effect relationship in which mechanical friction on the cornea causes oxidative stress and the production of damaging free radicals within the corneal tissue which may result in corneal thinning.30,36 This has been documented in repeated case reports in which patients who rubbed their eyes for reasons including ocular allergy, atopy, compulsive or nervous personality disorder, punctal agenesis (and subsequent chronic watery eye), stress or dry eye subsequently developed keratoconus.1-3,30,32-36 As such, it is the responsibility of eye care practitioners to keep modifiable risk factors in mind and educate all patients about the adverse effects of such behaviors. If possible it may also be necessary to educate family members or care providers about the importance of eliminating eye rubbing, as many patients will rub their eyes habitually and sometimes without awareness that they are doing so.
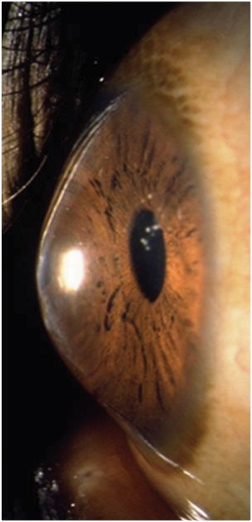 | |
| Anterior segment photo of keratoconus. Photo: Patrick Caroline, Pacific University College of Optometry. |
• Ultraviolet Light Exposure. Free radicals, a byproduct of oxidative stress, are formed with exposure to ultraviolet (UV) light or when mechanical insult occurs in a tissue. As compared to unaffected corneas, keratoconic corneas have been found to have more damaging byproducts.37 Additionally, keratoconus prevalence and severity has been reported as highest in the Middle East, though no sun exposure study has been performed. It is notable to consider the role of UV in the prevention of keratoconus progression, as it is used to activate riboflavin for corneal collagen crosslinking, which has been shown to strengthen bonds in the cornea.
Keratoconus is historically thought to be a noninflammatory disease process that involves thinning of the cornea. However, recent literature from a variety of sources suggests that due to increased MMP and other low-grade inflammatory markers, keratoconus may have an inflammatory component that results in damage to the structural integrity of the cornea. Because of its noninvasive nature, tear film analysis will remain a topic of great interest for the identification of biomarkers present to potentially aid in the etiology and early diagnosis of keratoconus.17
Discussion about possible relationships between keratoconus and sleep apnea, diabetes or hormonal changes during pregnancy are also emerging in a number of studies. It remains highly likely that there is a genetic predisposition to keratoconus, which can be triggered by environmental factors related to oxidation. The progressive etiological elucidation of keratoconus and other corneal ectasias will require continued research efforts by vision scientists and clinicians.
Dr. Morrison is the cornea and contact lens resident at Pacific University College of Optometry. She specializes in medical contact lenses and has a current research interest in gas permeable contact lens design, scleral shape and contact lens solutions.
1. American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern Guidelines. Corneal Ectasia. San Francisco, CA: American Academy of Ophthalmology; 2013. Available at: www.aao.org/ppp.
2. Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-69.
3. Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: A review. Contact Lens & Anterior Eye. 2010;33:157-66.
4. Kanellopoulous AJ, Asimellis G. OCT corneal epithelial topographic asymmetry as a sensitive diagnostic tool for early and advancing keratoconus. Clin Ophthalmol. 2014;18(8):2277-87.
5. Rad AS, Jabbarvand M, Saifi N. Progressive kerectasia after laser in situ keratomileusis. J Refract Surg. 2004;20(5):718-22.
6. Said A, Hamade IH, Tabbara KF. Late onset corneal ectasia after LASIK surgery. Saudi J of Ophthalmol. 2011;25:225-30.
7. Randeman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267-75.
8. O’Keefe M, Kirwan C. Laser epithelial keratomileusis ectasia in 2010 – a review. Clin Experiment Ophthalmol. 2010;38:183-91.
9. Schweitzer C, Roberts CJ, Mahmoud AM, et al. Screening of forme fruste keratoconus with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2010;51:2403-10.
10. Moshirfar M, Edmonds J, Behunin N, Christiansen SM. Corneal biomechanics in iatrogenic ectasia and keratoconus: A review of the literature. Oman J Ophthalmil. 2013;6(1):12-17.
11. Sachdev G, Sachdev MS, Sachdev R, Gupta H. Unilateral corneal ectasia following small-incision lenticule extraction. J Cataract Refract Surg 2015;41(9):2014-18.
12. Maatta M, Vaisanen MR, Pihlajaniemi T, Tervo T. Altered expression of type XIII collage in keratoconus and scarred human corneas: increased: increased expression in scarred human corneas is associated with myofibroblast transformation. Cornea. 2006;25:448-53.
13. Sugar J, Macsai MS. What causes keratoconus? Cornea. 2012;31:716-19.
14. Balasubramanian SA, Pye DC, Wilcox MD. Are proteinases the reason for keratoconus? Invest Ophthalmol Vis Sci. 2011;52:8592-7.
15. Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation an innate immunity. Nat Rev Immunol. 2004;4(8):617-29
16. Albert SJ, Cope L, Speck C, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS ONE. 2011;6(1):e16437.
17. Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye. 2015;29:843-59.
18. Romero-Jimenez M, Santodomingo R, Wolffsohn JS. Keratoconus: A review. Contact Lens Anterior Eye. 2010;33:157-66.
19. Weed KH, MacEwen CJ, McGhee CN. The variable expression of keratoconus within monozygotic twins: Dundee university Scottish keratoconus study (DUSK). Contact Lens Anterior Eye. 2006;29:123-26.
20. Bechera SJ, Shin JA, Newlin A, et al. Discordance for keratoconus in two pairs of monozygotic twins. Cornea. 1999;18:444-51.
21. Fullerton J, Paprocki P, Foote S, et al. Identify-by-descent approach to gene localization in eight individuals affected by keratoconus from north-west Tasmania, Austrialia. Hum Genet. 2002;110:462-70.
22. Bisceglia L, De Bonis P, Campo PA, et al. Linkage analysis in keratoconus: replication of locus 5q21.2 and identification of other suggested loci. Invest Ophthalmol Vis Sci. 2005; 46:39-45.
23. Liskova P, Ebenezer ND, Hysi PG, et al. Molecular analysis of the VSX1 gene in familial keratoconus. Mol Vis. 2007;4:1887-91.
24. Tang YG, Picornell Y, Su X. et al. Three VSX1 gene mutations, L159M, R166W, H244R, are not associated with keratoconus. Cornea. 2008;27:189-92.
25. Wang Y, Rabinowitz YS, Rotter JI, Yang H. Genetic epidemiology study of keratoconus: evidence for major gene determination. Am J Med Genet. 2000;93:403-09.
26. Gondhowiardjo TD, van Haerington NJ. Corneal aldehyde dehydrogenase, glutathione reductase, and glutathione S-transferase in pathologic corneas. Cornea. 1993;12:310-14.
27. Behndig A, Karlsson GK, Johannson BO, et al. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39:471-75.
28. Kenney MC, Brown DJ. The cascade hypothesis of keratoconus. Contact Lens Anterior Eye. 2003;26:139-46.
29. American Academy of Allergy, Asthma & Immunology. Conditions Dictionary. Available at: www.aaaai.org/conditions-and-treatments/conditions-dictionary.
30. Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-56.
31. Gordon-Shaag A, Millodot M, Essa M, et al. Is consanguinity a risk factor for keratoconus? Optom Vis Sci. 2013;90:448-54.
32. Wagner H, Barr JT, Zadnik K. Collaborative longitudinal evaluation of keratoconus (CLEK) study: methods and findings to date. Contact Lens & Anterior Eye. 2007;30:223-32.
33. Batool J, Lichter H, Stulting R. Asymmetric keratoconus attributed to eye rubbing. Cornea. 2004;23:560-64
34. Kandarakis A, Karampelas M, Soumplis V et al. A case of bilateral self-induced keratoconus in a patient with Tourette syndrome associated with compulsive eye rubbing: case report. BMC Ophthalmology. 2011;11:28.
35. Lindsay RG, Bruce AS, Gutteridge IF. Keratoconus associated with continual eye rubbing due to punctual agenesis. Cornea. 2000;19:567-69.
36. Krachmer JH. Eye rubbing can cause keratoconus. 2004;23:539-40.
37. Kenney MC, Chwa M, Atilano SR et al. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: Evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol and Vis Sci. 2005;46:823-32.
38. Rabinowitz YS, Wistow G. Gene expression profile studies of human keratoconus cornea for NEIBank: a novel cornea-expressed gene and the absence of transcripts for aquaporin 5. Invest Ophthalmol and Vis Sci. 2005;46:1239-46.


