 Uncomfortable contact lens wear continues to challenge clinicians as we strive to provide lasting relief for our symptomatic patients. With more than half of drop outs citing discomfort as the reason, it’s not surprising that more than half of the remaining contact lens wearers report discomfort with their lenses as well.1,2
Uncomfortable contact lens wear continues to challenge clinicians as we strive to provide lasting relief for our symptomatic patients. With more than half of drop outs citing discomfort as the reason, it’s not surprising that more than half of the remaining contact lens wearers report discomfort with their lenses as well.1,2
We know that successful contact lens wear hinges on a number of factors, including the health of the ocular surface and the tear film, both of which directly interact with the lenses, lens design and material, the condition of the lens surface, and contact lens care. By identifying underlying diseases that may predispose patients to lens wear problems, we can more proactively treat ocular discomfort associated with contact lenses. In this month’s column, we will analyze new technologies that may change the way we identify and monitor dry eye patients and more importantly, help lens wearers with comfort issues.
Osmolarity Testing
Many factors can affect the quality of the tear film. The mucin layer acts as the foundation of the tear film, which allows a smooth spreading of the aqueous layer over the ocular surface. The lipid layer, which is produced by the meibomian glands, is the most superficial layer of the tear film and acts as an anti-evaporative layer. Deficiencies in any of these layers can affect the integrity of the tear film and result in a number of the clinical signs and symptoms typically seen in dry eye patients. Unfortunately, many of the tests that we utilize clinically often show variable sensitivity and specificity in our dry eye population.
One measure that shows promise in providing a more sound, objective measure of the tear film quality is osmolarity. Osmolarity is the measure of solute concentration, defined as the number of osmoles of solute per liter of solution (Osms/L). In terms of the tear osmolarity, the units utilized are mOsms/L. Traditionally, this was done with a tear specimen in a lab that took about 15 minutes to measure. But, TearLab has produced a commercially available product that measures osmolarity within seconds from a 50nL sample collected from the inferior lateral tear meniscus (figure 1).
The increase of osmolarity has been shown to be a significant risk factor in the pathophysiology of dry eye.3,4 An increased concentration of electrolytes in the tear film is implicated as the reason for higher levels of osmolarity. An expert consensus on dry eye has confirmed that osmolarity is an important consideration in dry eye disease.3
A recent study examined a number of clinical markers and compared them to dry eye severity when trying to predict the level of dry eye disease.5 The researchers tested schirmer scores, tear film break up time (TBUT), ocular surface disease index (OSDI), corneal staining, conjunctival staining and tear film osmolarity. Patients were divided into one of three categories based on the severity of disease: normal, mild/moderate and severe. Of all the tests performed, only osmolarity showed a significant correlation to disease severity, especially in the mild moderate cohort.
Another study demonstrated that patients using artificial tears over a one-month time period were able to reduce their tear osmolarity readings.6 These findings suggest that measuring osmolarity is critial in diagnosis and management of dry eye and discomfort in lens wear.
Tear Prism
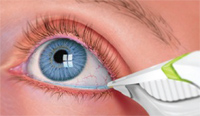
1. Collecting a tear sample directly from the eye allows practitioners to instanly measure osmolarity.
With the recent advancements in imaging technology, we now have the ability to more accurately measure tear prism. Tear prism is easily measured during the course of a typical examination with the help of fluorescein dye. A tear prism of 0.3mm or more is considered normal.
Spectral domain OCT has allowed us to image the eye in ways that were not previously possible. In a recent study, Zhenying and Xiaomei looked at tear prism measurements and their relationship to contact lens material.7 In this study, patients were randomized to either wear a hydrogel (hilafilcon B) or a silicone hydrogel (balafilcon A) contact lens for a month. Then, after a one-month washout period, patients wore the other lens.
The tear meniscus area (TMA), which was defined as the cross-sectional area of the tear prism, was measured using spectral domain OCT technology while patients wore both the hydrogel and silicone hydrogel lenses. The TMA was shown to be greater after a month of silicone hydrogel contact lens wear. Sensation of dryness was not as severe when the silicone hydrogel lenses were worn. Measuring tear prism may prove to be a clinically useful initial screening for those who are interested in contact lenses as well as for monitoring current contact lens wearers.
Meibum Expression
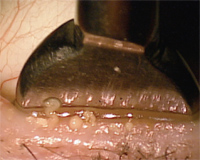
2. An example of gland expression utilizing the Mastrota paddle.
Properly functioning meibomian glands are critical to comfortable contact lens wear. Meibum that comes from a healthy meibomian gland has the consistency of a free-flowing fluid, very similar to vegetable oil. But in the abnormal state, meibum has a thicker consistency. In extreme cases of meibomian gland dysfunction and posterior blepharitis, stagnation of the oils in the glands may ensue.
Clinically, we assess the quality of these oils by applying pressure to the outside of the lid, close to the meibomian gland orifice. Some will role a cotton swab on the outside of the eyelid toward the eyelid margin in an attempt to express meibum. In cases where the oils are difficult to express, some practitioners will apply an anesthetic and compress the lids between two cotton-tip applicators.
Initially made of borosilicate glass, the Mastrota paddle is designed to offer a flat surface in which the palpebral surface of the lid rests while digital pressure is applied to the outside of the lid. The Mastrota paddle became commercially available in 2007 and is now made of titanium. The design of the paddle allows the practitioner to easily leverage the eyelid and express stagnated glands without needing to apply excessive force to the globe or cause iatrogenic irritation to the palpebral conjunctiva from cotton-tipped applicators (figure 2). Diagnostically, it allows the practitioner to assess the quality of the meibum. Expression will likely provide some therapeutic effect for those patients with a lipid deficient dry eye.
Here’s to the Future
We have a multitude of options to utilize in the diagnosis and management of our contact lens wearers. Incorporating these new tools and technologies into your contact lens examinations will improve patient care and ultimately lead to more comfortable contact lens wear.
Drs. Brujic and Miller would like to thank David Eldridge of TearLab Corp and Katherine Mastrota for their insights and assistance with this article.
1. Young G, Veys J, Pritchard N, Coleman S. A multi-centre study of lapsed contact lens wearers. Ophthalmic Physiol Opt. 2002 Nov;22(6):516-27.
2. Nichols JJ, Ziegler C, Mitchell GL, Nichols KK. Self-reported dry eye disease across refractive modalities. Invest Ophthalmol Vis Sci. 2005 Jun;46(6):1911-4.
3. The Ocular Surface. (DEWS Report). Available at: www.tearfilm.org/dewsreport/pdfs/TOS-0502-DEWS-noAds.pdf. (Accessed July 2010).
4. Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009 Aug;50(8):3671-9.
5. Foulks GN, Lemp MA, Berg M, et al. TearLab osmolarity as a biomarker for disease severity in mild to moderate dry eye disease. Poster presented at AAO 2009.
6. Benelli U, Nardi M, Posarelli C, Albert TG. Tear osmolarity measurement using the TearLab Osmolarity System in the assessment of dry eye treatment effectiveness. Cont Lens Anterior Eye. 2010 Apr;33(2):61-7.
7. Zhenying J, Xiaomei Q. Short-Term Effects of Silicone Hydrogel Wear on the Tears. Cont Lens Spect. 2010 Jan;26-33,44.


