Keratoconus patients typically experience wide-ranging visual fluctuations and disease progression from onset, typically at puberty, until the fourth decade of life, during which the condition becomes relatively stabilized. Though symptoms of visual aberrations are unwelcome at any point during one’s lifetime, the manifestations during these formative years are especially inopportune.
While refractive correction with gas permeable contact lenses is often the standard of care in keratoconus, management during the condition’s progressive years necessitates frequent contact lens adjustments, resulting in a significant reduction in quality of life and a substantial long-term economic burden for affected patients.1 According to conventional evidence, up to 20% of cases may go on to require penetrating keratoplasty.2 To date, despite steady advancements in contact lens technologies, keratoconus still remains the most common cause for such surgical intervention.
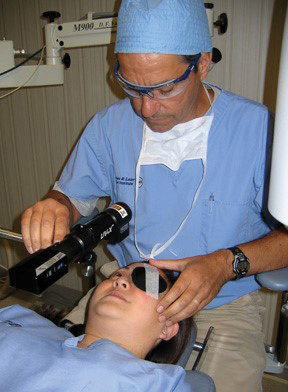 | |
| Fig. 1. Peter Hersh, MD, of the Cornea and Laser Eye Institute performing the conventional (i.e., Dresden) corneal crosslinking procedure. |
What if these patients could have their vision stabilized years or even decades earlier? Such is the game-changing potential that corneal crosslinking (CXL) represents. Despite having only recently obtained FDA clearance in April 2016, CXL with ultraviolet-A (UVA) and riboflavin photosensitizer has already been implemented around the world as a first-line treatment for keratoconus since the late 1990s (Figure 1).3–6 Its ability to stiffen corneal tissue makes CXL the first treatment to stabilize underlying stromal weakness and halt or slow corresponding progression of ectasia. Additionally, a global Delphi panel comprised of representatives from each of the four supranational corneal societies—the Asia Cornea Society, the Cornea Society, EuCornea and PanCornea—recently published a consensus report that recognized the importance of incorporating CXL as part of the new standard of care in managing keratoconus and ectatic diseases.7
Corneal Biomechanics Influence Shape
The ideal outcome for a patient with keratoconus is to arrest the condition’s continual progression before visual function is compromised. Hence, the potential benefits of early intervention with corneal crosslinking have sparked resurgent clinical interest in exploring diagnostic instrumentation that could more easily allow for early disease detection.
Recent investigative work in the field of corneal biomechanics may hold the potential for earlier identification of patients who could benefit from CXL, and could also aid in the analysis of CXL outcomes to further improve treatment parameters. These research findings have also provided insights into the implications of redistributing biomechanical stress in the cornea, with the intention of not only stabilizing the disease but also normalizing corneal shape.10-13
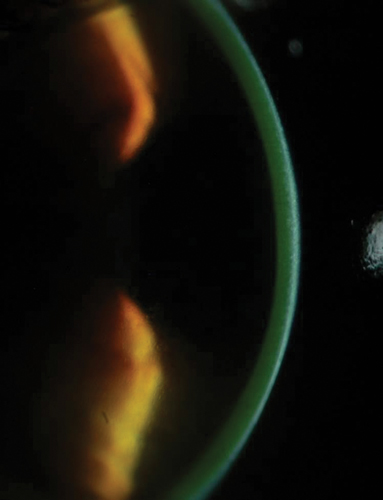 | |
| Fig. 2. Slit lamp photograph illustrating riboflavin in the corneal stroma following the loading phase. | |
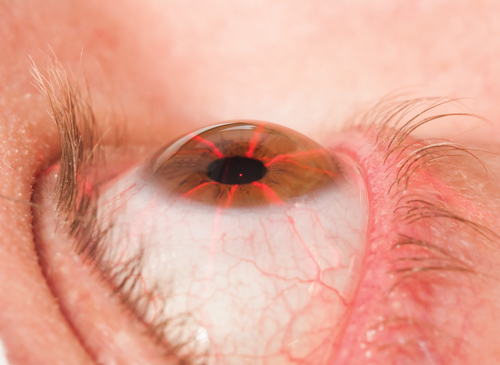 | |
| Fig. 3. The laser crosshairs of the KXL crosslinking system (Avedro) positioned to align the optical head of the system with the patient’s eye. |
The stiffness of the human cornea relies upon the lamellar organization of the stromal collagen fibers, which are regulated by an interconnecting network of proteoglycans.8 While there are still unanswered questions about the precise combination of molecular, genetic and environmental factors that contribute to the pathogenesis of keratoconus, it is believed that the interaction of these factors leads to the loss or slippage of collagen fibrils and changes to the extracellular matrix in the corneal stroma.9
Studies using x-ray scattering techniques reveal a disorganization of the collagen lamellae in the region of the cone, with a more normal organization of collagen in the surrounding regions.10 While studies from the 1970s and 1980s revealed bulk abnormalities in mechanical strength of keratoconic corneas relative to normal eyes, more recent work based on biomechanical modeling and Brillouin optical microscopy further demonstrates that these abnormalities may be attributed to focal weakening over the region of the cone, rather than across the entire cornea.11-13
The focalized reduction in elastic modulus within the affected corneal region deforms to a greater extent than the surrounding tissue when subjected to the strain of the normal intraocular pressure, manifesting in the conical protrusion. A proposed biomechanical cycle of decompensation suggests that the initial pathological changes triggered by genetic predisposition and environmental factors result in this focal weakening of the cornea. The initial asymmetry in elastic modulus is thought to initiate a cycle of stress redistribution in which the focal thinning results in increased biomechanical stress, leading to deformation and a further increase in stress, driving disease progression.11 If such theories are borne out by future research, a comprehensive assessment of corneal stress distribution would be essential to understanding the biomechanical factors at play in the progression of the ectasia and the efficacy of treatment options.
Currently, two commercially available devices measure corneal biomechanical properties by analyzing corneal behavior in response to a pulse of air. The Ocular Response Analyzer (Reichert) measures the difference in tissue response between in- and outgoing applanation pressures to provide a measurement of energy loss due to viscous damping. This clinical parameter is represented as corneal hysteresis (CH). Another device, the CorvisST (Oculus), uses a high-speed Scheimpflug camera to evaluate the deformation of the cornea in response to these pressures.14
Though both of these systems provide information about the overall biomechanical properties of the cornea, neither is able to map the regional differences in these properties. As such, the sensitivity of the devices may be insufficient to diagnose early or forme fruste keratoconus, due to the overlap in the range of values for normal and weakened corneas. Additionally, the absence of spatial information limits the applications of these systems in determining focal weakening of the cornea or measuring regional effects of corneal crosslinking within a treated cornea.14
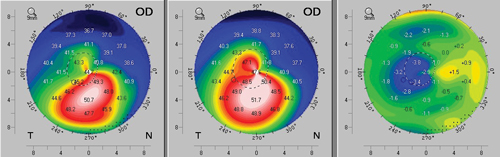 | |
| Fig. 4. A case example of topographic flattening after corneal crosslinking. Preoperative axial topography is shown at left, three-month postoperative topography at center, and a difference map revealing the change between the two time points is shown at right. | |
 | |
| Fig. 5. Customized, dual-arc UVA treatment pattern applied to the central cornea of a patient with intracorneal ring segments, using the KXL II system. |
Several techniques are in development to address the potential benefit of spatial measurement of corneal tissue properties, including supersonic shear imaging, Brillouin optical microscopy and optical coherence elastography. All hold promise to aid in the development of patient-specific corneal crosslinking procedures through improved understanding of regional differences in corneal stiffness.
Crosslinking Parameters Impact Clinical Outcome
When crosslinking was first introduced, a single treatment approach was applied in all cases. Termed “the Dresden protocol,” this conventional technique is performed following removal of the central 7mm to 9mm of epithelium. The stroma is saturated with riboflavin for 30 minutes, then irradiated with 365μm UVA at 3mW/cm2 for another 30 minutes for a total UVA dose of 5.4J/cm2 (Figure 2). Additional riboflavin drops are instilled at five-minute intervals during the irradiation phase.15 Several randomized, controlled trials have demonstrated statistically significant improvement in maximum keratometry (Kmax) or cone apex power in CXL-treated eyes compared with the untreated controls.16,17 Progression of Kmax of 2D or more has been observed in 0% to 4.3% of CXL-treated eyes.16-18 A long-term study of patients treated with the conventional protocol reveals persistence of the treatment effect through a 10-year follow-up period.19
Though further investigation is still necessary, significant advancements have been made in the last decade in regards to understanding the photochemical mechanisms that result in the formation of new crosslinks in the cornea. Under the right conditions, the interaction of UVA and riboflavin sets off a complex chain of photochemical reactions, resulting in the formation of covalent bonds within the intracellular matrix of the collagen lamellae, which effectively stiffens the cornea in the treated zone.5 This increase in stiffness may break the cycle of biomechanical weakening that results in ectasia, further limiting progression of the disease.11
With an improved understanding of corneal biomechanical behavior and the mechanisms of CXL comes a wave of new clinical efforts aimed at optimizing the procedure’s treatment parameters. Namely, this ongoing research will focus on deriving new CXL delivery protocols to decrease treatment time, increase both intra- and postoperative comfort, and improve efficacy, which could result in more meaningful corneal reshaping in addition to disease stabilization.
One such modification is the introduction of transepithelial crosslinking, which is intended to improve patient comfort and minimize infection risk.20 While standard formulations containing 0.1% riboflavin and 20% dextran show minimal penetration through an intact epithelium, new riboflavin formulations with added corneal-enhancing compounds like BAC and/or EDTA, can improve riboflavin diffusion despite epithelial presence.21-23 Attempts to disrupt the epithelial tight junctions without debriding the epithelium have also been carried out using disruptive devices, surgical sponges or iontophoretic delivery through the use of a mild electrical current.24-26
Another procedural modification is the use of accelerated CXL, which uses higher irradiance UVA (7mW/cm2 to 45mW/cm2) to shorten the amount of time necessary to deliver the equivalent total energy dose (Figure 3). Clinical studies evaluating the efficacy of accelerated CXL demonstrate stability or flattening of Kmax comparable to results achieved with the Dresden protocol.27,28 However, some investigators have examined the stromal demarcation lines following accelerated CXL—interpreted as an indirect indicator of the relative CXL treatment depth, commonly observed in the first one to three months post-op—and reported a trend towards shallower depth of the line following accelerated vs. conventional techniques.27,29-32
This difference in the demarcation line depth may be modulated by oxygen bioavailability within the stroma. Oxygen levels in the cornea are depleted by the photochemical reactions of CXL, with rapid oxygen replenishment once the UVA source is removed.33
One method to increase oxygen concentration in the cornea during accelerated CXL is to program the UVA emission to turn on and off at repeated time intervals to allow for diffusion of oxygen into the stroma during pauses in UV exposure.34 Hence, pulsed irradiation may have the potential to increase the corneal stiffening effect obtained with the same UVA dose, and may potentially lead to a reduction in procedure time by increasing the treatment efficiency of high irradiance CXL. Preliminary results with pulsed irradiation are promising, indicating safety equivalent to the continuous UV exposure protocols, albeit with greater demarcation line depth.35,36 However, longer follow-up is necessary to determine whether demarcation line depth significantly impacts clinical outcomes.
Interestingly, this correlation between the depth of the demarcation line and the different CXL protocols applied suggests a possible opportunity to customize CXL treatment parameters to target a specific depth—for example, to accomplish a shallower CXL effect in cases of thinner corneas by varying irradiance, total dose or pulse interval.37
Customized Crosslinking to Reshape the Cornea
Though corneal flattening and visual improvement have been reported, the primary goal of conventional CXL protocols is in fact to stabilize the cornea against progression and prevent further visual loss (Figure 4). Conventional CXL achieves this effect by uniformly stiffening the central 9mm of the anterior stroma. With the new understanding of the distribution of biomechanical forces within the keratoconic cornea comes the potential to customize crosslinking treatment plans based on an individual patient’s characteristics and corneal topography to achieve not just stabilization but also improvement in corneal shape.
In addition to varying the depth of crosslinking by modifying parameters like UVA irradiance, total energy dose and/or pulse interval, the lateral distribution of crosslink formation in the cornea may be better controlled through the use of customized UVA illumination patterns that would allow surgeons to focally stiffen the weakest region of the cornea rather than the conventional approach of uniformly stiffening the entire central cornea.12 Three-dimensional modeling of this concept suggests a potential for greater normalization of the cornea (inferior flattening and superior steepening), presenting the opportunity to combine visual rehabilitation and biomechanical stabilization into a single procedure. Photorefractive intrastromal corneal crosslinking (PiXL) is the clinical product of this theoretical approach.
PiXL uses a topographically customized UVA pattern to induce a variable distribution of crosslinking in the stroma (Figure 5). The CXL treatment depth (Z-axis) is controlled by the UVA energy parameters, while the lateral aspect (X-axis and Y-axis) is controlled by the specific UVA pattern applied using a crosslinking system containing a digital micromirror device (a semiconductor comprised of adjustable microscopic mirrors that can create a desired beam profile).38 The first clinical case report of PiXL application in a keratoconus patient used the KXL II System (Avedro) and demonstrated improvement in uncorrected visual acuity from 20/40 to 20/25 and reduction in corneal astigmatism of 0.8D at six months post-op.39
Though PiXL is not yet available in the United States, a number of ongoing studies in Europe are evaluating its potential. Several groups presented preliminary results at the 11th International Congress of Corneal Cross-linking in December 2015. Mazzotta used confocal microscopy to demonstrate the spatial distribution of the crosslinking treatment effect in response to various treatment protocols, while Cassange and Behndig presented the preliminary results of two prospective evaluations of PiXL compared to conventional CXL that demonstrated statistically significant improvement in keratometric parameters and visual acuity with PiXL.40-42 Further evaluations and longer follow-up will be needed to determine the optimum parameters for customized corneal crosslinking using the PiXL technique, however.
CXL has revolutionized keratoconus management by targeting underlying corneal instability and successfully stopping or slowing down disease progression. This innovation has energized research efforts to better understand the mechanisms driving keratoconus progression and to develop new diagnostic instrumentations to allow for earlier diagnosis. In addition, the next generation of CXL protocols is expected to attempt to further normalize the irregular contour of treated corneas and potentially improve patients’ visual function.
The author thanks Grace Lytle, OD, for her contributions to this article. Dr. Lytle is employed by Avedro, the manufacturer of the KXL crosslinking system.
Dr. Chang is the director of clinical services at TLC Laser Eye Centers. He has been a subinvestigator in numerous clinical studies and has published extensively on keratoconus treatment. He is also an advisory board member of the International Keratoconus Academy for Eye Care Professionals (IKA), the Gas Permeable lens Institute (GPLI), and the Optometric Cornea, Cataract and Refractive Society (OCCRS).
1. Rebenitsch RL, Kymes SM, Walline JJ, Gordon MO. The lifetime economic burden of keratoconus: a decision analysis using a markov model. Am J Ophthalmol. 2011;151(5):768-773.e2.
2. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319.
3. Chan E, Snibson GR. Current status of corneal collagen cross-linking for keratoconus: a review. Clin Exp Optom. 2013;17(Figure 2):1-10.
4. Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2009.
5. Meek KM, Hayes S. Corneal cross-linking - a review. Ophthalmic Physiol Opt. 2013;33(2):78-93.
6. Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul Surf. 2013;11(2):65-74.
7. Gomes JAP, Tan D, Rapuano CJ, et al. Global Consensus on Keratoconus and Ectatic Diseases. Cornea. 2015;34(4):359-369.
8. Lewis PN, Pinali C, Young RD, et al. Structural interactions between collagen and proteoglycans are elucidated by three-dimensional electron tomography of bovine cornea. Structure. 2010;18(2):239-245.
9. Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46(6):1948-1956.
10. Meek KM, Boote C. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog Retin Eye Res. 2009;28(5):369-392.
11. Roberts CJ, Dupps WJ. Biomechanics of corneal ectasia and biomechanical treatments. J Cataract Refract Surg. 2014;40(6):991-998.
12. Roy AS, Dupps WJ. Patient-specific computational modeling of keratoconus progression and differential responses to collagen cross-linking. Invest Ophthalmol Vis Sci. 2011;52(12):9174-9187.
13. Scarcelli G, Besner S, Pineda R, Yun SH. Biomechanical characterization of keratoconus corneas ex vivo with brillouin microscopy. Invest Ophthalmol Vis Sci. 2014;55(7):4490-4495.
14. Girard MJA, Dupps WJ, Baskaran M, et al. Translating Ocular Biomechanics into Clinical Practice: Current State and Future Prospects. Current eye research. 2015;40(1):1-18.
15. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627.
16. Wittig-Silva C, Chan E, Islam FM et al. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812-821.
17. O’Brart DPS, Kwong TQ, Patel P, et al. Long-term follow-up of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linking to halt the progression of keratoconus. Br J Ophthalmol. February 2013.
18. Chang CY, Hersh PS. Corneal collagen cross-linking: a review of 1-year outcomes. Eye Contact Lens. 2014;40(6):345-352.
19. Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: Ten-year results. J Cataract Refract Surg. 2015;41(1):41-46.
20. Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon M-J. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38(6):1000-1005.
21. Hayes S, O’Brart DP, Lamdin LS, et al. Effect of complete epithelial debridement before riboflavin-ultraviolet-A corneal collagen crosslinking therapy. J Cataract Refract Surg. 2008;34(4):657-661.
22. Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009;35(5):893-899.
23. Raiskup F, Pinelli R, Spoerl E. Riboflavin Osmolar Modification for Transepithelial Corneal Cross-Linking. 2012;37(September 2010):234-238.
24. Rechichi M, Mazzotta C, Daya S, et al. Epithelial disruption pulsed accelerated cross-linking : one year results. In: 10th CXL Congress 2014 Zurich; 2014.
25. Stojanovic A, Chen X, Jin N, et al. Safety and efficacy of epithelium-on corneal collagen cross-linking using a multifactorial approach to achieve proper stromal riboflavin saturation. J Ophthalmol. 2012;2012:498435.
26. Cassagne M, Laurent C, Rodrigues M, et al. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with riboflavin in a rabbit model. Invest Ophthalmol Vis Sci. 2014;33(0):1-6.
27. Tomita M, Mita M, Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014;40(6):1013-1020.
28. Mita M, Waring GO, Tomita M. High-irradiance accelerated collagen crosslinking for the treatment of keratoconus: Six-month results. J Cataract Refract Surg. 2014;40(6):1032-1040.
29. Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea. 2006;25(9):1057-1059.
30. Mazzotta C, Caporossi T, Denaro R, et al. Morphological and functional correlations in riboflavin UV A corneal collagen cross-linking for keratoconus. Acta Ophthalmol. April 2010:1-7.
31. Touboul D, Efron N, Smadja D, et al. Corneal Confocal Microscopy Following Conventional, Transepithelial, and Accelerated Corneal Collagen Cross-linking Procedures for Keratoconus. J Refract Surg. 2012;28(11):769-776.
32. Kymionis G, Tsoulnaras K. Corneal Stromal Demarcation Line Determined With Anterior Segment Optical Coherence Tomography Following a Very High Intensity Corneal Collagen Crosslinking. Cornea. 2015:664-667.
33. Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53(4):2360-2367.
34. Kamaev P, Eddington W, Rood-Ojalvo S, et al. Accelerated corneal cross-linking with pulsed light. Invest Ophthalmol Vis Sci. 2013;54(E-Abstract 5288).
35. Mazzotta C, Traversi C, Caragiuli S, Rechichi M. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond). 2014;28(10):1179-1183.
36. Mazzotta C, Traversi C, Paradiso AL, et al. Pulsed Light Accelerated Crosslinking vs Continuous Light Accelerated Crosslinking: One-Year Results. J Ophthalmol. 2014:1-15.
37. Friedman MF, Smirnov M, Kamaev P, Mrochen M, Lytle G, Muller D. Can we safely cross-link thinner corneas: Pathways for optimized CXL treatment planning. European Society of Cataract & Refractive Surgery Annual Meeting, Barcelona, Spain, September 3, 2015. Poster.)
38. Lytle G. Advances in the Technology of Corneal Cross-Linking for Keratoconus. Eye Contact Lens. 2014;0(0):1-7.
39. Kanellopoulos AJ, Dupps WJ, Seven I, Asimellis G. Toric topographically customized transepithelial, pulsed, very high-fluence, higher energy and higher riboflavin concentration collagen cross-linking in keratoconus. Case Rep Ophthalmol. 2014;5(2):172-180.
40. Mazzota C. Biological reations after CXL.Paper Presented at: 11th International Congress of Corneal Cross-Linking; 2015 Dec 4-5; Boston, MA.
41. Behndig A. Clinical results with PiXL in keratoconus Eyes. Paper Presented at: 11th International Congress of Corneal Cross-Linking; 2015 Dec 4-5; Boston, MA.
42. Cassagne M. Clinical results with PiXL in keratoconus Eyes. Paper Presented at: 11th International Congress of Conreal Cross-Linking; 2015 Dec 4-5; Boston, MA.


