Since the first corneal transplant was performed more than one hundred years ago, this vision-restoring procedure has been performed more than one million times.1 For the vast majority of these procedures, corneal transplant surgery involved replacing the entire thickness of the area being transplanted, even when most of the tissue remained healthy. While versions of an anterior lamellar keratoplasty were introduced in the 1950s, and dissection of Descemet’s membrane was attempted in the 1970s, “deep lamellar keratoplasty,” as it is used today, did not become a viable alternative until the late 1990s.2,3 At this time, problems with penetrating keratoplasty (PK) surgery included risk of rejection, a high degree of irregular astigmatism, as well as cataracts and glaucoma, which often resulted from the need to use steroids for prolonged periods of time to reduce the risk of rejection.4-6 But, by leaving a portion of the host cornea in place, all of these risks were lessened—at least, in theory. Yes, PK was a risky procedure, but the difficulty was developing predictable alternatives that provide visual outcomes equivalent to those of PK.
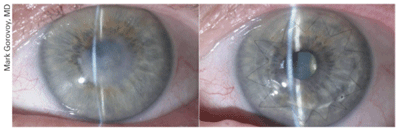
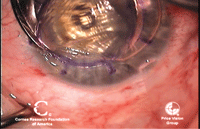
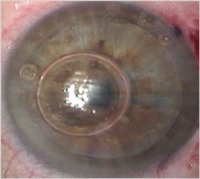
1a. This preoperative cornea is
scarred from a corneal ulcer. 1b. Postoperative DALK. DALK is a viable
option in this case as the scarring is limited to the anterior layers
of the cornea. Photo Courtesy: Mark Gorovoy, M.D.
Today, the percentage of full-thickness corneal transplant procedures continues to decline. In 2008, over one-third of all corneal grafts were endothelial keratoplasties, and the actual number of endothelial keratoplasties has tripled since 2006.7 Some surgeons are doing lamellar grafts in more than 90% of their corneal transplant patients.8 There is less risk of rejection and other complications with LK, and recent developments in technique result in visual outcomes more comparable to those of PK.9,10
With the recent developments in deep lamellar keratoplasty, those who are not active in corneal surgery comanagement may be left confused. So, in this article, we’ll analyze the differences between techniques and outcomes of PK and LK, in an effort to enhance the reader’s understanding of deep lamellar surgeries.
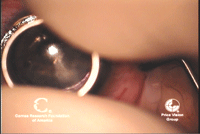
2.
A trephine is used to create a deep cut in the host
cornea—approximately 350µm to 400µm—which will determine the size of
the graft and allow access to the deep stroma. Photo Courtesy: Francis
Price, M.D.
Deep Anterior Lamellar Keratoplasty (DALK)
Of the many layers of the cornea, the endothelial layer is the most immunogenic. This can be the most significant source of risk of rejection in penetrating keratoplasty procedures.11 In cases where the disease or damage is limited to the stroma, the affected portions of the anterior cornea can be removed, saving the native endothelial layer. The initial problems with DALK were poor visual outcomes due to irregularity of the dissected surfaces and scarring in the tissue interfaces; however, by the late 1990s—particularly with the advent of air or liquid dissection of the layers—the procedure had evolved to the point where visual outcomes were comparable to those of PK.12 Yet, this technique was complex and tedious, limiting its use.
DALK can be utilized in almost any situation where the endothelium and Descemet’s membrane are healthy, but the overlying stroma or Bowman’s membrane is damaged, resulting in decreased vision. The most common conditions for which DALK can be effective are keratoconus, corneal scarring (figure 1), corneal dystrophies and ocular surface conditions that lead to corneal damage.13
3, 4. A channel is created in
the deep stroma to facilitate the separation of layers with air or
fluid (left). A cannula is used to inject air or viscoelastic into the
deep layers of stroma, creating a smooth separation of the stromal
layers (right). Photos Courtesy: Francis Price, M.D.
There are various techniques for performing DALK. There is a general distinction between DALK procedures that are pre-Descemetic (those that do not fully remove all the stroma) vs. those that are Descemetic (those that fully reach Descemet’s membrane). For optimal visual results, reaching Descemet’s membrane is desirable, and the main concern with going as deep as possible is the increased risk of perforation.14
The basic DALK technique involves removing the corneal tissue in two basic steps. The first step is to remove the majority of the anterior cornea. The process begins with a partial trephination approximately 350µm to 400µm deep (figure 2). Once the cut has been made, the anterior corneal tissue may be separated manually from the underlying tissue and cut off. The next step is to insert a dissector to the desired depth of the graft, usually as near Descemet’s membrane as possible (figure 3). Once the dissector has created a space, a cannula is inserted, and air or viscoelastic is injected to separate the layers smoothly (figure 4). Once the air bubble is injected, the surgeon removes the remaining anterior cornea by simply cutting it off (figure 5).
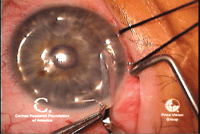
5. Once the stromal layers are
dissected, the anterior cornea is removed with surgical scissors. Note
the scissors on the left side of the image cutting away the anterior
cornea.
6. The corneal graft is laid in place and sutured. Photos Courtesy: Francis Price, M.D.
In an alternate DALK technique, the surgeon inserts the dissector to create a pocket at the desired depth immediately after the trephination and injects the air or viscoelastic at this point. The procedure is the same, though further trimming of the cornea tissue may be required if the necessary depth is not achieved by the initial bubble.
After the remaining stroma is trimmed off, leaving a smooth surface of Descemet’s membrane, the eye is ready for the donor tissue. Descemet’s membrane and the endothelium are peeled away from the donor tissue, and then the remaining cornea is placed atop the host cornea, marked and sutured into place (figures 6 and 7).
The IntraLase Femtosecond (FS) laser has application in DALK procedures just as it does in full-thickness grafts. The IntraLase can make zig-zag and box cuts to both the donor and recipient tissue so that the tissues interlock, strengthening and stabilizing the transplant.
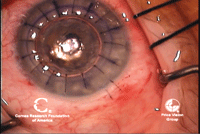
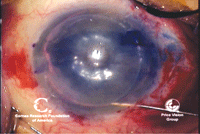
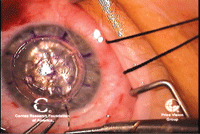
7, 8. DALK at completion (left).
Note air bubble in anterior chamber. The air bubble indicates
Descemet’s membrane is intact. An Intralase-assisted PK graft with host
junction (right). Note the zig-zag cuts that interlink. Photo Courtesy:
Francis Price, M.D.
Although it is possible to use the FS laser in DALK, it may be more valuable in PK, where wound strength can be a significant issue. Wound strength may be enhanced in PK by using the FS laser to create interlocking edges. Ultimately, because wound strength is inherently greater in LK procedures vs. penetrating procedures, the FS laser may be of less value in DALK.
In recent years, the statistical evidence increasingly supports DALK as a better procedure for certain patients. Risk of rejection is lower in DALK than in PK, and the risk of developing glaucoma is lower in DALK as well.12,14,15 Endothelial cell density is also better for those undergoing DALK.15 Some studies report less keratometric astigmatism with DALK than PK, while others suggest that results are comparable.14,16 DALK also allows for the use of less robust tissue, as higher endothelial cell counts are not required for this procedure.
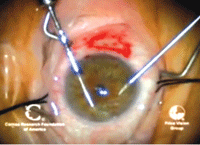
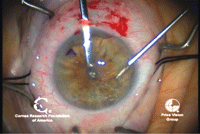
9, 10. In DSAEK, Descemet’s
membrane is scored with a Sinsky hook (left). The endothelium and
Descemet’s membrane are removed (right). Photos Courtesy: Francis
Price, M.D.
The current disadvantages of DALK are the learning curve for surgeons, the risk of perforation (which may require conversion to a full PK), interface opacification, and non-resolving creases in Descemet’s membrane—this requires a back-up graft, which puts demands on the local eye bank. In some cases, it is possible that perforation will not require switching to a full graft, but it is a possibility.
Posterior Lamellar Keratoplasty
While the terminology for anterior lamellar keratoplasty is straightforward, there has been an “alphabet soup” of acronyms for posterior lamellar procedures—e.g., DLEK, DSEK, DSAEK or DMEK. The first official term to describe these posterior procedures was coined by Gerrit Melles, M.D., a pioneer in lamellar keratoplasty techniques, and associates in 1998: posterior lamellar keratoplasty (PLK). The evolution of these techniques has been more significant than on the anterior side, and a host of variations on this technique spawned myriad acronyms.
11. The DSAEK donor is prepped with a microkeratome. Photo Courtesy: Mark Gorovoy, M.D.

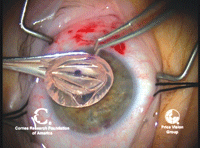
12.
The donor tissue, having already been cut with a microkeratome, will be
separated from the anterior cornea, have a drop of viscoelastic placed
on the endothelium and folded in half for insertion. Photo Courtesy:
Francis Price, M.D.
The main reasons for posterior lamellar keratoplasty are diseases of the endothelial layer, such as bullous keratopathy, Iridocorneal Endothelial (ICE) Syndrome, Fuch’s corneal dystrophy, one of the many other less common endothelial corneal dystrophies (e.g., posterior polymorphous dystrophy), or a failed graft with an otherwise good refractive outcome.17
• Deep Lamellar Endothelial Keratoplasty. Unlike original attempts at endothelial transplants, which involved creating an anterior flap of tissue, trephining the damaged endothelium out and suturing the donor tissue in, DLEK preserved the preoperative corneal surface in an attempt to maintain a normal corneal topography.
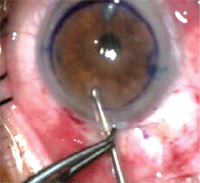
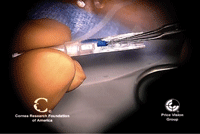
13. This folded endothelial graft is being loaded with a modified Gossey forceps. Photo Courtesy: Mark Gorovoy, M.D.
14. This endothelial graft is properly positioned and held in place with an air bubble. Photo Courtesy: Francis Price, M.D.
Originally, DLEK began with the creation of a 9.0mm scleral wound, approximately 1mm to 2mm posterior to the superior corneal limbus. This opening was large enough to insert a special trephine that cut through the endothelium, Descemet’s membrane and the posterior stroma. Special scissors would then be used to resect the diseased host tissue. The wound was closed with a single temporary safety suture, and the microscope was turned to the donor table, where the donor cornea was then prepared by cutting it down to a depth of 150µm via manual dissection.18 The posterior donor disc would be placed endothelial side down onto a Healon-coated spatula and brought into the patient’s anterior chamber, which is filled with air. The donor tissue is raised anteriorly until the donor stromal surface and the recipient stromal bed surface contact, and then the spatula is withdrawn from the eye on a layer of Healon, leaving the donor tissue in place, supported by the air bubble in the anterior chamber and self-adhering without sutures.18
The problems with this approach stem from the 9.0mm scleral wound size, as well as the interface between the donor and host stroma.
The visual outcomes for DLEK are generally in the 20/40 to 20/50 range.19-21 While DLEK was not the long-term solution for endothelial keratoplasty, it began moving corneal surgery in the direction of increasingly less invasive and more anterior cornea-sparing techniques. It has been displaced for the majority of cases by DSEK/DSAEK.22
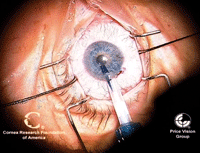
15, 16. The trypan blue-stained
endothelial graft is loaded for insertion (left). The endothelial graft
is loaded into the recipient anterior chamber (right). Photos Courtesy:
Francis Price, M.D.
• DSEK and DSAEK. In 2003, Dr. Melles proposed a procedure for stripping Descemet’s membrane from the back of the recipient cornea. In this procedure, Descemet’s is stripped in much the same fashion that a capsulorhexis is done in cataract surgery, and is often referred to as a Descemetorhexis. The advantages of this vs. DLEK with a trephine are numerous and include smaller incision surgery and all the advantages that go along with that, as well as a smoother recipient interface with the new tissue. Stripping Descemet’s membrane from the posterior stroma leaves a smoother surface than dissecting the stromal layers. This procedure became known as Descemet’s stripping endothelial keratoplasty (DSEK).
A further evolution of this procedure utilizes a microkeratome for separating the posterior layers of the donor (not recipient) cornea, providing a smoother interface than manual dissection. This procedure is known as Descemet’s stripping automated endothelial keratoplasy (DSAEK).
The only difference between these procedures is the manner in which the donor cornea tissue is prepared for transplantation—manually in DSEK and via keratome in DSAEK. Using a keratome provides a smoother surface, which translates to better visual outcome, so DSAEK is preferred by a majority of surgeons today.22,23
To initiate the procedure, the surgeon creates limbal paracentesis incisions and maintains the anterior chamber space with either an irrigating anterior chamber maintainer or with a viscoelastic device. The cornea is marked for reference points, the main operating wound is enlarged, and a blunt inverted Sinsky hook is inserted into the anterior chamber. Descemet’s membrane is scored for 360º in the corneal mid-periphery. The diseased endothelium and Descemet’s membrane of the recipient is easily punctured by the blunt tip of the reverse Sinsky hook.24
Once the diameter of the recipient bed has been scored, an angled Descemet’s stripper is used to gently peel Descemet’s membrane away from the stroma and strip it off from the central posterior cornea (figure 10). The periphery of the recipient bed may then be scraped in order to aid in later donor adherence. At this time, anterior corneal venting incisions can be made to later remove any fluid in the interface between the graft and the host. The viscoelastic is removed with an irrigation/aspiration tip.24
The donor tissue is prepared either by manual dissection or with a Moria microkeratome (figure 11), leaving endothelium, Descemet’s membrane and a section of posterior stroma. This tissue, usually 8.0mm to 9.0mm in diameter, is folded in half to facilitate passage through the 4mm to 5mm incision.24 A small drop of viscoelastic is placed on the endothelium for protection during the tissue folding (figures 12 and 13). Once in the anterior chamber, the tissue is unfolded and air is injected to raise the tissue into contact with the posterior stroma. A firm air bubble presses the graft into place for about eight to 10 minutes, during which time the venting incisions are drained. Most of the air is then removed and replaced with balanced salt solution, and a smaller air bubble will stay in the eye for several hours to stabilize the graft (figure 14).
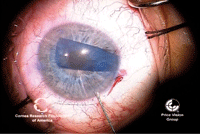
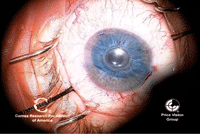
17, 18. The endothelial graft
is inserted into the anterior chamber (left). An air bubble is injected
under the graft to aid in the unfolding and unrolling of the graft
(right). Photos Courtesy: Francis Price, M.D.
There are many advantages to a DSAEK over a PK—namely reduced risk of rejection, much more rapid visual recovery and minimal induced astigmatism from the procedure.25 The timeframe for recovery from a posterior lamellar transplant is often weeks as opposed to months, which is a significant factor for patients considering corneal surgery.
There are really no significant disadvantages for these procedures, assuming proper patient selection. Ideal candidates would be patients with diseased endothelium for nearly any reason, with an otherwise normal cornea. Contraindications would include chronic hypotony, anterior corneal opacities, keratoconus and those with anterior chamber IOLs.23 There are risks in the procedures unique to DSAEK and not to PK, such as graft dislocation, but there are many other risks that are reduced or eliminated by not making a full-thickness trephination.25 In some cases, the interface between the graft and the host can account for a reduction in the best-corrected visual acuity to the 20/25 or 20/30 level.
• Descemet’s Membrane Endothelial Keratoplasty (DMEK). DMEK is the most recent development in posterior lamellar keratoplasty, and it holds the potential for better visual outcomes than even DSAEK.26
DMEK involves removing the diseased posterior cornea in a similar fashion to DSEK and DSAEK, but Descemet’s membrane and the endothelium are transplanted with little or no posterior stroma, as opposed to DSEK or DSAEK, in which up to 150µm of posterior stroma is often transplanted.27 Eliminating the posterior stroma enables surgeons to achieve better visual outcomes, but the technique has not been completely perfected at this point. Graft dislocation rates remain high, and predictability and the minimization of tissue loss are still being improved.28
DMEK begins with the same process of endothelial removal as DSEK and DSAEK (figures 9 and 10). The donor preparation is simpler than in DSAEK, as a microkeratome and artificial anterior chamber are not required. But, the process is complex nonetheless due to the delicate nature of the tissues. The endothelium can be marked, and Descemet’s membrane of the donor tissue scored. A circular Descemet’s membrane-endothelial graft can then be gently dissected free using forceps. The graft is then stained with Trypan blue.29 The host cornea is also prepared in a similar fashion to DSAEK. The donor Descemet’s-endothelial graft is partially rolled and placed in an injector system (figure 15), which injects the tissue into the anterior chamber (figures 16 and 17). The tissue is unrolled with an air bubble, which pushes it upward into the recipient bed (figures 18 - 20).
By removing the posterior stroma from the graft, DMEK can provide the optimal visual outcome of any posterior lamellar keratoplasty. Recovery is as quick and easy as with any lamellar graft. Removal of the donor endothelium does not require a microkeratome, which can translate into a significant cost savings. Unlike DSAEK and DSEK, which can cause hyperopic refractive shifts due to the retained stromal tissue, the refractive status of the eye is as unchanged as possible.30
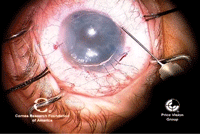
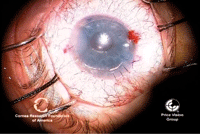
19, 20. Once
the graft is unrolled, more air is injected to push the graft up to the
host stroma (left). Right, DMEK is complete, with the endothelial graft
in good position. Photos Courtesy: Francis Price, M.D.
But, DMEK is not without disadvantages: The donor tissue prep and the manipulation of the tissue are far more challenging for the surgeon than in DSAEK, and the dislocation rate is significantly higher than in DSAEK, while the visual outcomes are often only marginally better. That said, DMEK may be the posterior lamellar procedure of the next decade. As the technique evolves and the risks and complications are reduced, DMEK may displace DSAEK as the predominant posterior lamellar procedure.
A Matter of Choice
DALK is a useful procedure, particularly in parts of the world where high-quality grafts are less frequently available than others. It may also be a preferable procedure for patients whose risk of rejection may be higher or where optimal postoperative care is not as readily available. DSAEK and DSEK have become widely accepted as preferred procedures for patients with posterior corneal disease and no other complicating factors. Assuming the same rate of growth in number of endothelial keratoplasty procedures as the years 2005 through 2008, DSAEK and DSEK rates may be approaching 50% of all corneal grafts by 2011.7 DMEK, while in the developmental stages, pushes visual outcomes to their highest current potential and offers the fastest recovery, while challenging the corneal surgeon with a more delicate procedure.
All of these procedures certainly have their place in the corneal surgeon’s arsenal of treatment options for patients with visually significant corneal disease.
Dr. Jedlicka is the Director of Contact
Lens Services at the University of Minnesota Department of
Ophthalmology and a partner at Consultative Eye Care, a referral-based
practice in Wayzata, Minn. He is a clinical adjunct faculty member at
the Pennsylvania College of Optometry, Salus University, and lectures
on contact lens and anterior segment-related topics. Dr.
Awad is a board-certified fellowship-trained eye surgeon specializing
in cornea and external diseases, cataract and refractive surgery. He
practices in the Minneapolis-St. Paul area.
1. Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin Experiment Ophthalmol. 2005 Dec;33(6):642-57.
2. Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea. 2002 May;21(4):374-83.
3. Trimarchi F, Poppi E, Klersy C, Piacentini C. Deep lamellar keratoplasty. Ophthalmologica. 2001 Nov-Dec;215(6):389-93.
4. Kirkness CM, Ficker LA, Steele AD, Rice NS. The success of penetrating keratoplasty for keratoconus. Eye (Lond). 1990;4 ( Pt 5):673-88.
5. Rumelt S, Blum-Hareuveni T, Bersudsky V, Rehany U. Development and progression of cataract in patients required repeated corneal transplantation. Eye (Lond). 2003 Nov;17(9):1025-31.
6. Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U. Preexisting and postoperative glaucoma in repeated corneal transplantation. Cornea. 2002 Nov;21(8):759-65
7. Eye Bank Association of America, 2008 Statistical Report, April 2009.
8. Newer techniques in lamellar surgery, Eyeworld, Jan. 2010.
9. Busin, M. DSAEK for the treatment of endothelial disease: results in the initial 100 cases Klin Monbl Augenheilkd. 2009 Sep;226(9):757-60.
10. Han DC, Mehta JS, Por YM, et al. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009 Nov;148(5):629-31.
11. Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981 Apr;99(4):599-604.
12. Trimarchi F, Poppi E, Klersy C, Piacentini C. Deep lamellar keratoplasty. Ophthalmologica. 2001 Nov-Dec;215(6):389-93.
13. Shimmura S, Tsubota K. Deep Anterior Lamellar Keratoplasty. Curr Opin Ophthalmol. 2006 Aug;17(4):349-55.
14. Shimazaki J, Shimmura S, Ishioka M, Tsubota K. Randomized clinical trial of deep lamellar keratoplasty vs penetrating keratoplasty. Am J Ophthalmol. 2002 Aug;134(2):159-65.
15. Han DC, Mehta JS, Por YM, et al. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009 Nov;148(5):744-751.e1.
16. Funnell CL, Ball J, Noble BA. Comparative cohort study of the outcomes of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Eye (Lond). 2006 May;20(5):527-32.
17. Melles GR, Eggink FA, Lander F, Pels E, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998 Nov;17(6):618-26.
18. Terry MA. Deep lamellar endothelial keratoplasty (DLEK): pursuing the ideal goals of endothelial replacement. Eye. 2003;17:982–88.
19. Fogla R, Padmanabhan P. Initial results of small incision deep lamellar endothelial keratoplasty (DLEK). Am J Ophthalmol. 2006 Feb;141(2):346-351.
20. Terry MA. Endothelial replacement: the limbal pocket approach. Ophthalmol Clin North Am. 2003 Mar;16(1):103-12.
21. Terry MA, Ousley PJ. Rapid visual rehabilitation with deep lamellar endothelial keratoplasty. Cornea. 2004 Mar;23(2):143-53.
22. Bahar I, Kaiserman I, McAllum P, et al. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008 Sep;115(9):1525-33.
23. Dapena I, Ham L, Melles GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK—the thinner the better? Curr Opin Ophthalmol. 2009 Jul;20(4):299-307.
24. Endothelial keratoplasty challenges the primacy of full-thickness transplantation, Available at: www.osnsupersite.com/view.aspx?rid=60693. (Accessed March 2010).
25. Prepare for hyperopic shift following DSAEK graft surgery. Ophthalmology Times. 33(19):1.
26. New techniques change indications and outcomes of keratoplasty. Ocular Surgery News. Available at: www.osnsupersite.com/view.aspx?rid=12422. (Accessed March 2010).
27. Price FW, Price M. DSEK: What You Need to Know About Endothelial Keratoplasty. Slack, Inc: Thorofare, NJ. 2009.
28. Terry M, Hoar K. Descemet’s stripping automated endothelial keratoplasty (DSAEK): Techniques, tips and ways to prevent dislocation. Available at: www.cnpg.com/vide/651/Descemet’s-Stripping-Automated-Endothelial-Keratoplasty-(DSAEK)-Techniques,-Tips-And-Ways-to-Prevent-Dislocation.aspx. (Accessed March 2010).
29. Dalton M. From PKP to DMEK: An update on corneal transplant techniques. EyeWorld. Available at: www.eyeworld.org/article.php?sid=4445&strict=0&morphologic=0&query=.(Accessed March 2010).
30. Busin M. DMEK latest in selective tissue corneal transplantation surgery. Ocular Surgery News. Available at: www.osnsupersite.com/view.aspx?rid=32722. (Accessed March 2010).


