When fitting patients with contact lenses, eye care practitioners utilize their expertise to choose lenses with attributes that will best meet each patient’s vision and eye health needs. Silicone hydrogel (SiHy) lenses offer greater oxygen transmissibility than hydrogel lenses, which is one reason their market share has grown to more than 70% of all contact lens fits and refits in the United States.1,2 Once the lens has been chosen, should practitioners go one step further and prescribe a contact lens solution? Absolutely.
A patient who is not prescribed a contact lens solution or receives no explanation as to why a certain solution is best is unprepared to make an informed decision when confronted with an entire store aisle of options. An ill-informed patient may resort to picking a product based solely on the sale price or available coupons. Choosing paper towels based on price may not have long-term consequences, but choosing a contact lens solution using this method can.
To underscore the importance of their recommendation, practitioners should write solution recommendations on a prescription pad, as would be done with a prescription eye drop. Remember that we have many variables to consider when it comes to contact lens solutions, such as soak time, efficacy of disinfection, comfort and biocompatibility. Practitioners should consider the solution’s ability to clean/disinfect the contact lens and increase the patient’s comfort with the prescribed lenses. In addition to comfort and disinfection, there are other patient considerations that practitioners need to address, as they can also impact the contact lens-wearing experience, such as price and ease of use.
This article will review the attributes of some of the most commonly used contact lens solutions, and how their use may impact the contact lens-wearing patient—particularly those wearing SiHy lenses.
Patients are always concerned about price, even more so in these economic times. Those concerned with the cost associated with contact lens solution use may switch, without the practitioner’s knowledge, to a lower-cost solution that may not have all of the attributes of the prescribed solution or one that may not be as effective with the prescribed lenses. If a more expensive contact lens solution is prescribed, the practitioner should explain the benefits the patient will derive from using that particular product. If the cost of a brand name solution is prohibitive, the practitioner should offer alternative private label solutions.
Private label solutions are safe to use. However, just like their brand name counterparts, they have different product attributes that make some solutions more appropriate for certain patients. Inform your patients that chemical makeup of private label solutions may change periodically without a noticeable change in the bottle/package appearance. This is why we must instruct patients to read the ingredients label to guarantee that there are no solution changes since the last purchase.
Another impact of price is that patients may start rationing their contact lens solution. Not using the recommended amount of solution or topping off old solution can lead to inadequate lens disinfection and an increased risk for eye infection.3 Practitioners need to communicate to the patient the importance of using the prescribed contact lens solution, and using it as directed.
 Is it Easy to Use?
Is it Easy to Use?
Contact lens solutions can be divided into two groups: multipurpose solutions (MPS) that use a rub/rinse or a rinse-only method and hydrogen peroxide (H2O2) solutions that use a rinse-only method. An MPS that requires patients to rub takes approximately a minute longer than rinsing alone and can lead to substantially cleaner lenses than the rinse-only method.4,5 Cleaner lenses can result in clearer vision and greater comfort. Making patients aware of the great benefits of the rub/rinse step requires minimal extra effort, but these recommendations may go a long way to ensuring compliance and thus successful lens wear.
Multipurpose solutions that have been FDA cleared as rinse-only and continue to be marketed as “No Rub” are effective when used according to the manufacturer’s guidelines; this entails that the patient rinse both sides of each lens for at least five seconds per side, which many patients fail to do. It is recommended that patients rub even with a “No Rub” MPS.
The FDA, American Optometric Association and American Academy of Ophthalmology all recommend that patients using an MPS utilize the rub/rinse method.6,7 In response to the FDA recommendation and the fact that about 92% of practitioners recommend that patients use a rub/rinse regimen, Bausch + Lomb has revised its directions for use of FDA-cleared rinse-only products to recommend a rubbing step.8
Hydrogen peroxide solutions require a rinse, and not just the soak, in order to effectively clean lenses. Unlike lenses cleaned using an MPS, lenses cleaned using an H2O2 solution must be neutralized prior to insertion into the eye.9 If this step is not performed, the patient will suffer chemical keratitis upon lens insertion. The number of patient reports of chemical keratitis due to H2O2 solutions on the FDA’s Manufacturer and User Facility Device Experience (MAUDE) site suggests that these painful experiences are not uncommon occurrences.10
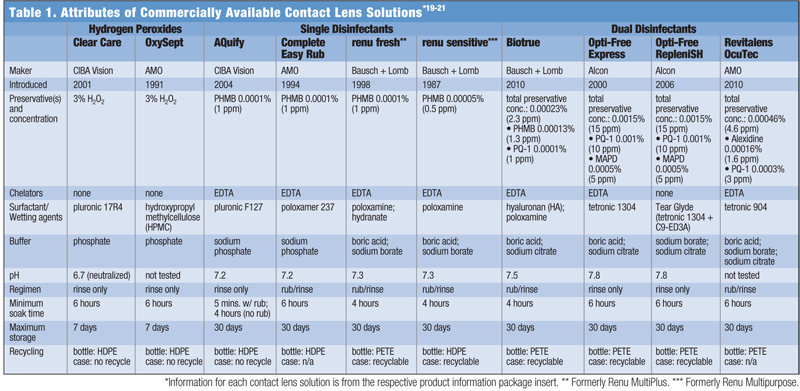
Soak time is another attribute that can impact ease of use. Most MPS and H2O2 solutions require a six-hour soak, but some MPS only require as little as four hours. One solution, AQuify Multi-purpose Solution (CIBA Vision), has an express rub option that allows the patient to soak lenses for as little as five minutes (see “Attributes of Commercially Available Contact Lens Solutions”).
Lens care is not difficult and one system is not necessarily easier for the patient to use properly than another. Whether the practitioner decides to prescribe an MPS or H2O2 solution, the main challenge lies in the need for the solution to be used consistently and as directed. To this end, patient education is of vital importance and must be reinforced at every opportunity. Again, highlighting the benefits of compliance—better comfort and crisp vision—can demonstrate to the patient that there is more to be gained from lens care compliance than simply minimizing the risk of infection. The practitioner should explain to the patient that ease of use does not translate to lack of importance.
Make Comfort a Priority
A host of variables can affect comfort with contact lens wear. Contact lens solutions can help keep lenses comfortable from the time of insertion through the end of day via the use of humectants, lubricants and surfactants. These agents keep lenses lubricated and modify the lens surface to reduce its impact on the ocular environment when inserted. Surfactants also contribute to keeping lenses clean.11-13 Contact lens solutions utilize different combinations of agents to improve patient comfort with contact lens wear. A recent trend in new ocular products is to use nature for inspiration. One such product is an MPS that utilizes hyaluronan—a lubricant used in some rewetting drops that can be found naturally throughout the body. Hyaluronan is an interesting comfort agent for two reasons: first, one molecule can hold up to 1,000 times its weight in water; and second, in one study hyaluronan was shown to remain on SiHy lenses for up to 20 hours.14,15
All MPS contain preservatives and other excipients, to which some patients may demonstrate a level of sensitivity. For this small percentage of patients, it may be best to prescribe a peroxide-based lens cleaning system (see “Attributes of Commercially Available Contact Lens Solutions”).9
Contact lens solutions contain buffers to maintain pH levels and osmolarity agents for tonicity, both of which also play a significant role with regard to comfort. The purpose of including these agents in the solution formulation is to make these attributes as close in value as possible to those of the tear film. The pH range of healthy tear film is approximately 7.3 to 7.7 and the average osmolarity is 305 mOsm/kg.16-18 The greater the difference between the values for the solution and the tear film, the greater the reaction by the ocular environment upon lens insertion, which may manifest as a stinging sensation or irritation. The above chart lists the pH and buffers of various contact lens solutions.19-21
Disinfection and cleaning will be discussed in greater detail in a later section, but comfort can also be affected by the cleanliness of the lenses, as the existence of denatured protein deposits may reduce comfort.22 Patients should also be advised that noncompliance with the replacement schedule of their lenses can impact comfort, as shown in a study of patients wearing two-week or monthly replacement SiHy lenses.23
Discomfort is often cited as the main reason for patient discontinuation of contact lens wear.24-26 Practitioners can try to minimize discomfort by prescribing a contact lens solution that has shown to be effective in cleaning and lubricating the lenses prescribed. Being knowledgeable of the different lens formulations can also help the practitioner navigate through the numerous options to the one that provides the greatest benefits in cleaning and lubricating. Compliance with use of contact lens solutions and the recommended replacement frequency of contact lenses can also help minimize the risk of discomfort.
Keep it Clean and Disinfected
The most important attribute that practitioners consider when choosing a contact lens solution is its ability to disinfect lenses. A clean lens is the cornerstone of ocular health in contact lens-wearing patients. The rub/rinse method has been shown to remove more than 80% of microbial organisms from the contact lens surface and is the recommended method of regulatory and society bodies for cleaning contact lenses with an MPS.6,7,27 Some MPS are FDA cleared and continue to be marketed for the rinse-only method, but this method is not as effective as the rub/rinse method. This notion is confirmed by a study by Kwok Hei Mok, Ph.D., and colleagues where some rinse-only MPS were unable to remove protein deposits from the surface of soft contact lenses, and by a study by Andrew D. Pucker, B.S., and Jason J. Nichols, O.D., M.P.H., where use of a rinse-only solution left approximately 40% of deposited proteins on worn SiHy lenses.28,29 SiHy lenses are more prone to lipid deposits than protein deposits, but denatured proteins on the lens are more likely to lead to clinically significant issues such as contact lens-induced papillary conjunctivitis (CLPC).
All contact lens solutions meet the minimum FDA requirements for disinfection of Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Fusarium solani and Candida albicans, which are the standard American Type Culture Collection (ATCC) organisms tested. These organisms are not the only threats to contact lens wearers; clinical isolates harvested from worn contact lenses, used contact lens cases and infected eyes of the same species as FDA-tested organisms are often more difficult to kill than lab-adapted microbes.30 Methicillin-resistant S. aureus (MRSA) and Acanthamoeba are additional organisms that are also more difficult to kill than lab-adapted microbes. The challenge with killing MRSA is that it is already impervious to several antibiotics; while Acanthamoeba provides a challenge since it exists in two forms—as a cyst and as a trophozoite.31,32 These and other clinical isolates commonly found on lenses and on lens care accessories are not currently part of the FDA requirements for disinfection, and contact lens solutions have varying levels of success against them.33-37
The differing levels of disinfection by lens care solutions against these organisms depend not only on the type of disinfectants used but the overall formulation, including components such as chelators (i.e., EDTA) and buffers (i.e., boric acid), which play an integral role.11,19,38,39 MPS can use either single- or dual-disinfectant systems. Single-disinfectant systems contain PHMB. All dual-disinfectant systems are branded solutions, and they utilize the combinations polyquaternium-1 (PQ-1, Polyquad [Alcon]) and myristamidopropyl dimethylamine (MAPD, Aldox [Alcon]), PHMB and PQ-1, or alexidine and PQ-1. Peroxide-based solutions utilize 3% hydrogen peroxide to disinfect and neutralize bacteria, fungi, viruses and certain protozoa.9 These solutions must meet the same disinfection standards as the MPS.
Cleaning and disinfection are important to patients because they reduce their risk of infection—as well as non-infectious inflammatory reactions such as infiltrative keratitis—from contact lens wear, and protein deposits can reduce their comfort level. Patients who experience infections, inflammatory events or discomfort are more likely to discontinue wearing contact lenses. Compliance is key, which is why practitioners must educate their patients on how to clean their lenses, maintain and replace their lens cases and to follow expiration dates.6,40 Furthermore, practitioners may want to prescribe a solution/system that comes with a new lens case with each full-size bottle of lens care solution to increase the probability of patients replacing their cases at the recommended intervals. Prescribing the contact lenses and lens solution that will work best for the patient is the first step, ensuring that patients use them effectively is the second.
Maintaining Eye Health
To practitioners, eye health focuses on the absence of infection and maintaining the overall health of the cornea. The biocompatibility of the prescribed contact lenses and the prescribed contact lens solution with the eye warrants consideration. Clinical observations associated with disinfectants/preservatives are a common topic of discussion among practitioners. There is a fair amount of material on corneal staining in the literature; however, there is some debate over the clinical and statistical significance of the corneal staining observed with MPS at two hours.41-43 In addition, some studies have shown that what clinicians are seeing is not physiological or pathological corneal staining, but preservative-associated, transient hyperfluorescence (PATH)—a benign phenomenon.44,45 Hopefully ongoing and future studies will be able to clarify this issue.
Some contact lens solutions are more compatible with the eye than others, as demonstrated by cytotoxicity assays and clinical outcomes (e.g., contact lens-associated infiltrates/infiltrative keratitis).46-49 Some patients are more receptive to products that contain natural agents or are bio-inspired, as they are perceived to be less taxing on the eye.20
Patients who experience red eye or discomfort as a result of contact lens wear are more likely to discontinue wearing contact lenses, replace the prescribed contact lenses and lens care solutions with other options, or to start self-medicating with drops. Practitioners need to follow up with their contact lens patients to see if they are experiencing irritation; if so, ascertain if the patient is being compliant with the lens replacement schedule and the lens care regimen. If compliance is not the issue, practitioners should determine whether a change in lenses or solution is warranted, or if the culprit is actually an ocular surface disorder. It is important for practitioners to take the lead in recommending a strategy in order to ensure that the patient does not choose a course of action that may not only leave the original issue unresolved, but possibly create new and more serious ones.
Going Green
Some patients are very environmentally concerned and want to use a solution that comes in a recyclable bottle and can be used with a recyclable lens case. Municipalities that recycle plastic also recycle PETE, polyethylene terephthalate-based plastics. Some may also recycle HDPE, high-density polyethylene-based plastics. Most lens cases for MPS are made of polypropylene and are recyclable—the one exception is ProGuard (CIBA Vision). Lens cases for H2O2 solutions are not recyclable because they contain metal. Practitioners should remember to ask about the patient’s preferences on recyclable contact lens solution bottles and lens cases (see “Attributes of Commercially Available Contact Lens Solutions”).
Choosing the right contact lens solution for each patient is important, but compliance with the lens care regimen will dictate the level of success that is achieved. The practitioner should discuss the reasons a particular solution was chosen, so that the patient understands why it is important to not switch to a different solution. The benefits of keeping the lenses clean (i.e., clearer vision and greater comfort) should be communicated so that patients know to expect some tangible results for their efforts. By choosing the right contact lens solution for each patient, and working to keep the level of compliance with lens care high, the practitioner can help patients to achieve greater satisfaction with their contact lens wear.
Editorial support provided by BioScience Communications.
S. Barry Eiden, O.D., is the President and Medical Director of North Suburban Vision Consultant, Ltd., and is an assistant clinical professor at the University of Illinois, Chicago, Department of Ophthalmology, cornea and contact lens service. He is a Fellow of the American Academy of Optometry and is the immediate past chair of the Contact Lens and Cornea Section of the American Optometric Association.
1. Efron N, Morgan PB, Cameron ID, et al. Oxygen permeability and water content of silicone hydrogel contact lens materials. Optom Vis Sci. 2007 Apr;84(4):E328-E337.
2. Morgan PB, Woods CA, Tranoudis IG, et al. International contact lens prescribing in 2010. CL Spectrum. 2011 Jan. Available at:
www.clspectrum.com/article.aspx?article=105084 (Accessed March 2011).
3. Clayton-Jeter HD. Looking good: safe use and care of contact lenses. U.S. Food and Drug Administration. 2010 May. Available at:
www.fda.gov/forhealthprofessionals/articlesofinterest/ucm211838.htm (Accessed March 2011).
4. Johnson C, Kohler N, Bean B, et al. Rub vs. no rub: what are the costs? CL Spectrum. 2002 Jul. Available at: www.clspectrum.com/article.aspx?article=12170 (Accessed March 2011).
5. Butcko V, McMahon TT, Joslin CE, Jones L. Microbial keratitis and the role of rub and rinsing. Eye Contact Lens. 2007 Nov;33(6):421-3.
6. U.S. Food and Drug Administration. Ensuring Safe Use of Contact Lens Solution. 2009 Jun. Available at:
www.fda.gov/ForConsumers/ConsumerUpdates/ucm16+4197.htm (Accessed February 25, 2011).
7. American Optometric Association. Contact lenses. Available at:
http://www.aoa.org/x5230.xml (Accessed February 23, 2011).
8. Wu Y, Carnt N, Willcox M, Stapleton F. Contact lens and lens storage case cleaning instructions: whose advice should we follow? Eye Contact Lens. 2010 Mar;36(2):68-72.
9. Ward MA. Revisiting hydrogen peroxide disinfection. CL Spectrum. 2006 May. Available at:
www.clspectrum.com/article.aspx?article=13026 (Accessed March 2011).
10. U.S. Food and Drug Administration. MAUDE - Manufacturer and User Facility Device Experience. Available at:
www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. (Accessed March 2011).
11. Lin MC, Svitova TF. Contact lenses wettability in vitro: effect of surface-active ingredients. Optom Vis Sci. 2010 Jun;87(6):440-7.
12. Sindt CW. What’s in your blister pack? Rev Cornea Contact Lens. 2010 Jun;146(5):10.
13. Veys J, Meyler J, Davies I. Essential contact lens practice – Part 11 (C-8223). 2008 Jan. Available at:
www.opticianonline.net/Articles/2008/01/11/20157/Essential+contact+lens+practice+-+Part+11+(C-8223).html (Accessed March 2011).
14. Hargittai I, Hargittai M. Molecular structure of hyaluronan: an introduction. Struct Chem. 2008;19(5):697-717.
15. Scheuer CA, Fridman KM, Barniak VL, et al. Retention of conditioning agent hyaluronan on hydrogel contact lenses. Cont Lens Anterior Eye. 2010 Dec;33 Suppl 1:S2-6.
16. Moses RA. Adler’s Physiology of the Eye. 7th ed. St. Louis: The C.V. Mosby Company;1981.
17. Farris RL. Tear osmolarity – new gold standard? Adv Exp Med Biol. 1994;350:495-503.
18. Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. 2005 Apr;3(2):81-95.
19. Dalton K, Subbaraman LN, Rogers R, Jones L. Physical properties of soft contact lens solutions. Optom Vis Sci. 2008 Feb;85(2):122-8.
20. Venkatesh S. Biotrue multipurpose solution: bringing inspiration to lens care. Optician. 2010 Jan;30-6.
21. Bausch + Lomb, Inc. Data on file. Results are from pH comparison studies of three separate lots of Biotrue, CIBA Clear Care, AMO Complete Easy Rub, AMO RevitaLens OcuTec, Alcon Opti-Free Express, and Alcon Opti-Free RepleniSH. The pH of the test solutions was determined using a Fisher Scientific accumet excel XL25 pH meter. Five pH measurements for each solution were taken and averaged. 2010.
22. Henry VA. The role of compliance. Rev Cornea Contact Lens. 2009 Oct;146(7):27-32.
23. Dumbleton K, Woods C, Jones L, et al. Comfort and vision with silicone hydrogel lenses: effect of compliance. Optom Vis Sci. 2010 Jun;87(6):421-25.
24. Richdale K, Sinnott LT, Skadahl E, et al. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea. 2007 Feb;26(2):168-74.
25. Rumpakis J. New data on contact lens dropouts: an international perspective. Rev Optom. 2010 Jan;147(1):37-42.
26. Fonn D. Preventing contact lens dropouts. CL Spectrum. 2002 Aug. Available at:
www.clspectrum.com/article.aspx?article=12186 (Accessed March 2011).
27. Cho P, Cheng SY, Chan WY, Yip WK. Soft contact lens cleaning: rub or no-rub? Ophthal Physiol Opt. 2009 Jan;29:49-57.
28. Mok KH, Cheung RWL, Wong BKH, et al. Effectiveness of no-rub contact lens cleaning on protein removal: a pilot study. Optom Vis Sci. 2004 Jun;81(6):468-70.
29. Pucker AD, Nichols JJ. Impact of a rinse step on protein removal from silicone hydrogel contact lenses. Optom Vis Sci. 2009 Aug;86(8):943-47.
30. Hume EB, Flanagan J, Masoudi S, et al. Soft contact lens disinfection solution efficacy: clinical Fusarium isolates vs. ATCC 36031. Optom Vis Sci. 2009 May;86(5):415-9.
31. Centers for Disease Control and Prevention. Definition of MRSA. 2010 Aug. Available at:
www.cdc.gov/mrsa/definition/index.html (Accessed March 2, 2011).
32. Crum-Cianflone NF. Acanthamoeba. 2002 Jun. Available at:
www.emedicine.medscape.com/article/211214-print (Accessed April 30, 2010).
33. Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens. 2010 Mar;36(2):116-29.
34. Willcox MD, Carnt N, Diec J, et al. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci. 2010 Jul;87(7):456-64.
35. Abbott Medical Optics Inc. Data on file. Santa Ana, CA. Biocidal efficacy of COMPLETE RevitaLens MPDS against ISO 14729 test organisms. 2010.
36. Kilvington S, Nikolic M, Lam A, et al. Comparative antimicrobial efficacy of contact lens care solutions. Poster presented at: British Contact Lens Association’s 34th Clinical Conference and Exhibition, May 27-30, 2010; Birmingham, UK.
37. Melton R, Thomas R, Snyder C. Methicillin-resistant Staphylococcus aureus in eyecare and in the contact lens practice. CL Spectrum. 2010 Feb. Available at:
www.clspectrum.com/article.aspx?article=103932 (Accessed March 2011).
38. Nikolic M, Kilvington S, Cheung S, et al. Survival and growth of Stenotrophomonas maltophilia in multipurpose contact lens solutions. Presented at: The British Contact Lens Association 34th Annual Meeting, May 27-30, 2010; Birmingham, UK.
39. Koffler BH, Karpecki PM. Positive aspects of the use of multipurpose disinfection solutions. Arch Ophthalmol. 2009 Nov;127(11):1540-3.
40. British Contact Lens Association. The dos and don’ts of contact lens wear. Available at:
www.bcla.org.uk/en/consumers/consumer-guide-to-contact-lenses/the-dos-and-donts-of-contact-lens-wear.cfm (Accessed March 2, 2011).
41. Andrasko G, Ryen K. A series of evaluations of MPS and silicone hydrogel lens combinations. Rev Cornea Contact Lens. 2007 Mar:143(2):36-42.
42. Morgan PB, Maldonado-Codina C. Corneal staining: Do we really understand what we are seeing? Cont Lens Anterior Eye. 2009 Apr;32(2):48-54.
43. Dillehay SM, Long B, Cutter G. A statistical analysis of the staining grid. CL Spectrum. 2007 Nov. Available at:
www.clspectrum.com/article.aspx?article=101062 (Accessed March 2011).
44. Ikeda T, Ledwith A, Bamford CH, Hann RA. Interaction of a polymeric biguanide biocide with phospholipids membranes. Biochim Biophys Acta. 1984 Jan;769(1):57-66.
45. Bright FV, Maziarz P, Liu M, et al. Cell membrane integrity modeling with polyaminopropyl biguanide (PHMB) exposure using fluorescent spectroscopy and liposome assays. Poster presented at: The 6th Biennial Scientific Symposium of the Contact Lens Association of Ophthalmologists Education & Research Foundation, September 23-25, 2010; Las Vegas, NV.
46. Cavet ME, VanDerMeid KR, Harrington KL, et al. Effect of a novel multipurpose contact lens solution on human corneal epithelial barrier. Cont Lens Anterior Eye. 2010 Dec;33(Suppl 1):S18-23.
47. McCanna DJ, Harrington KL, Driot JY, et al. Use of a human corneal epithelial cell line for screening the safety of contact lens care solutions in vitro. Eye Contact Lens. 2008 Jan;34(1):6-12.
48. Carnt NA, Evans VE, Naduvilath TJ, et al. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch Ophthalmol. 2009 Dec;127(12):1616-23.
49. Kislan TP, Hom MM. Corneal infiltrates with multipurpose solutions and contact lens combinations. Poster presented at Annual Meeting of the Association for Research and Vision in Ophthalmology, May 2-6, 2010; Ft. Lauderdale, FL.


