Complaints against repeat offender dry eye syndrome, otherwise known by the alias ocular surface disease (OSD), keep coming into our optometric practices at an alarming rate. Today, OSD is estimated to be prevalent in 6% to 20% of the adult population––with some subgroups reporting much higher levels.1-4 And for most patients, OSD has a measurably negative impact on quality of life.5
Upon further investigation, we find that the treatment and management of OSD signs and symptoms often is unsatisfactory, especially with conventional therapy—e.g., tear supplementation and preservation. The alternative: More contemporary measures, such as anti-inflammatory therapies, nutritional supplementation and autologous serum eye drops, may show promise.
Target Inflammation
Anti-inflammatory therapies have become increasingly popular in first-line OSD treatment, particularly as our understanding of the significance of inflammation’s role in OSD evolves.6,7 A typical treatment regimen includes artificial tear supplementation, a several-week course of a topical steroid (dosed QID the first week to month, and then tapered to BID for up to two months)—a site-specific steroid like loteprednol 0.2% or 0.5% is ideal for this therapy—and a long-term course of twice-daily cyclosporine. Although generally considered an effective therapy, drawbacks to this approach include its relatively high cost, the risk of side effects from topical steroid use such as potential increased IOP, depressed immune system and delay in corneal wound healing, as well as complaints of stinging upon instillation of cyclosporine.6,8 While cyclosporine is FDA-approved for the treatment of dry eye caused by inflammation, the use of topical steroids for the treatment of dry eye is still considered off-label.
Nutritional Support
The role of nutritional supplementation, particularly omega-3 fatty acids, is well-established and becoming mainstream in optometric practices (see “A Supplemental Treatment”). However, practitioners stumble in building awareness of the potential benefits of omega-3-containing foods and supplements in their practices. Why? Many optometrists feel that they lack the depth of knowledge to make informed recommendations to their patients; others believe that there isn’t enough time in a routine eye exam to allow for nutritional counseling. It doesn’t help that the literature, while rich in quantity, is often contradictory regarding intake recommendations.
Before making any firm recommendations, it’s critical to understand your patient’s current food and supplement consumption. For example, an easy way to calculate omega-3 fatty acid intake is to ask how often your patient eats fish that is broiled, baked or grilled. The key is in the type of fish, as well as its preparation. Fried fish sticks do more harm than good, while grilled salmon is a nutritional winner. Patients who eat healthy fish three or more times per week are less likely to even have dry eye. For patients who eat fish that frequently, remember to recommend a lower supplemental intake than for those who rarely, if ever, eat healthy fish.
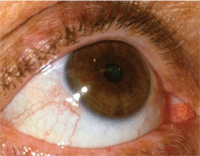
1. Severe dry eye can result in corneal epithelial disruption. Autologous serum preparations have been shown to restore corneal structural damage.
Initial supplementation should range from 1500mg and 3000mg per day, depending on fish intake, body weight (heavier patients should take a bit more than leaner patients), presence of other systemic diseases (hypertension, diabetes, hypercholesterolemia, hypertriglyceridemia and depression can benefit from higher intakes) and other lifestyle factors. While some experts may suggest lower (as low as 600mg per day) or even much higher (as high as 6,000mg per day) levels, the 1,500mg to 3,000mg is unlikely to yield adverse systemic side effects.9,10
Further, this intake range refers specifically to the supplement’s sum of EPA and DHA. These values usually are included near the bottom of the label, if they are included at all. (Note: Think twice before recommending any supplements without full nutritional labeling.) Ideally, the total amount of EPA+DHA shouldn’t be less than half of the total omega-3 fatty acids; most of the high-quality supplements have 75% to 80% or more EPA+DHA. If OSD is the only ailment being addressed with omega-3 fatty acid supplementation, and symptoms have improved, the intake can be reduced to the range of 1,000mg to 1,500mg (EPA+DHA) for maintenance therapy.8,9
Biologic Therapeutics
First introduced in the late 1980s, biologic therapeutics—products derived from the patient’s own whole blood (also known as autologous serum eye drops or platelet-rich plasma) are relatively new treatments for OSD.7,11
To create autologous serum drops, a patient’s blood is drawn, allowed to clot and then centrifuged to allow separation of serum from the whole blood sample. The serum is then diluted with saline or another appropriate product, filtered or sterilized, and placed into droppers to be used up to eight times a day.12 One session typically yields 100ml of blood, 30ml to 35ml of serum, and approximately a three-months supply of eye drops that are dosed six to eight times per day.
Serum eye drops must be kept frozen until needed, and any remaining drops must be discarded at the end of the day of use. Although it takes just a few hours to prepare the vials of tears, they are not dispensed until appropriate microbiological tests are concluded, to ensure they are safe for topical use.13
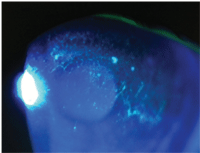
2. Patients with mild to moderate dry eye usually have mild injection and a tear film meniscus less than 0.5mm in height.
There are significant advantages to blood-derived eye drops. In dry eye disease, there is a lack of the so-called epitheliotrophic factors—substances in the tear film that promote proliferation and differentiation of ocular surface cells. These substances include fibronectin, vitamins and growth factors. Autologous serum contains similar biochemical properties, and is non-allergenic because it is derived from the patient’s own blood. Several clinical trials have shown that autologous serum drops are superior to artificial tear substitutes in ameliorating the signs and symptoms of OSD.14 When produced by a licensed physician for use in his or own professional practice, FDA registration or approval is not required for autologous serum eye drops––despite their classification as a medical product.13
Hormonal Therapy
Hormonal influences on the ocular surface have been under investigation for several years. Because of the increased prevalence of OSD in perimenopausal and postmenopausal women, estrogen was long believed to play an important role in maintenance of the ocular surface. However, androgens are now thought to play an even more essential role in promoting lacrimal gland, salivary gland and meibomian gland function.15-18 Dehydroepiandrosterone (DHEA) is a critical substance involved in maintenance of secretory glands; in estrogen-deficient individuals, there is a proportional deficiency in DHEA.
The result of several complex biochemical reactions is a disruption in the estrogen/androgen balance, which then can lead to autoantigen formation and ultimately auto-inflammatory and autoimmune disease.18 This may, in part, explain the pathogenesis in some Sjögren’s syndrome patients, where the ratio of occurrence in females is approximately 9:1.19 It would seem logical that supplementation with oral DHEA might marginalize the symptoms of Sjögren’s syndrome, but some studies have found limited or no benefit with this therapy.20,21
More recently, topical DHEA drops in varying concentrations are being used off-label with mixed anecdotal results. It should be noted that, to date, there is a lack of evidence from large-scale clinical trials for either oral or topical DHEA in treating dry eye associated with Sjögren’s syndrome.
Acupuncture
Acupuncture can hardly be considered a new therapy, but its appearance in Western literature regarding potential applications in OSD is relatively recent. Many studies suggest it can help improve the signs and symptoms of dry eye.22,23 Currently, there is no standard treatment protocol. Some practitioners report improvement after a single treatment, but long-lasting effects are elusive. One drawback in interpreting these results is that the methodology is not always consistent between studies. Further, because the nature of acupuncture is patient- and symptom-specific, it is difficult to replicate the precise methodology from one study to another. Nevertheless, many patients seek acupuncture as a complementary therapy and report positive results.
As with other acupuncture therapies, the theory of its mechanism of action in ameliorating the signs and symptoms of dry eye rests with a balancing of the autonomic nervous system. Specifically, acupuncture seems to provide a cholinergic anti-inflammatory effect by enhancing vagus nerve activity.24
Lifestyle
Finally, we should never underestimate the benefits of lifestyle and environmental changes and how they can enhance comfort in OSD patients. Some familiar examples include smoking cessation, adequate sleep, proper hydration and taking frequent breaks when performing near tasks (e.g., working on the computer).
As our understanding of the pathogenesis of ocular surface disease evolves, so do our options in treatment. Tear supplementation, nutrition, environmental modifications and anti-inflammatory therapies are established and have become the standard of care for dry eye sufferers. However, newer therapies like autologous serum eye drops, hormonal therapy and acupuncture may move into the mainstream as we continue to expand our knowledge in this area.
Dr. Reed is an associate professor at Nova Southeastern University College of Optometry in Fort Lauderdale, FL. She teaches and writes extensively about ocular disease, ocular pharmacology, and nutrition.
1. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009 Jun;127(6):763-8.
2. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003 Aug;136(2):318-26.
3. Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011 Sep;152(3):377-84.
4. Kosrirukvongs P, Ngowyutagon P, Pusuwan P, et al. Prevalence of dry eye syndrome and Sjogren’s syndrome in patients with rheumatoid arthritis. J Med Assoc Thai. 2012 Apr;95 Suppl4:S61-9.
5. Le Q, Zhou X, Ge L, et al. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol. 2012 Jul 16;12:22.
6. Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004 Sep;138(3):444-57.
7. Jarka ES, Kahrjoff M, Crane MB. Dry-eye—is inflammation just the tip of the iceberg? Optometry. 2012 Mar 30;83(3):111-3.
8. Mah F, Milner M, Yiu S, et al. PERSIST: Physician’s Evaluation of Restasis ®Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012;6:1071-6.
9. Wojtowica JC, Butovich I, Uchiyama E, et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011 Mar;30(3):308-14.
10. Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:336-56.
11. Fox RI, Chan R, Michelson JB, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1084 Apr;27(4):459-61.
12. Cho YK, Huang W, Kim GY, Lim BS. Comparison of Autologous Serum Eye Drops With Different Dilutants. Curr Eye Res. 2012 Aug 28.
13. Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467-74.
14. Nelson JD, Drake MM, Brewer JTJ, et al. Evaluation of a physiological tear substitute in patients with keratoconjunctivitis sicca. Adv Exp Med boil. 1994;350:453-7.
15. Sullivan DA, Belanger A, Cermak JM, et al. Are women with Sjogren’s syndrome androgen-deficient? J Rheumagol. 2003 Nov;30(11):2413-9.
16. Porola P, Laine M, Virkki L, et al. The influence of sex steroids on Sjogren’s syndrome. Ann N Y Acad Sci. 2007 Jun;1108:426-32.
17. Porola P, Birkki L, Przybyla BD, et al. Androgen deficiency and defective intracrine processing of dehydroepiandrosterone in salivary glands in Sjogren’s syndrome. J Rheumatol. 2008 Nov;35(11):2229-35.
18. Konttinen YT, Fuellen G, Bing Y, et al. Sex steroids in Sjogren’s syndrome. J Autoimmun. 2012 Aug;39(1-2):49-56.
19. Goëb V, Salle V, Duhaut P, et al. Clinical significance of autoantibodies recognizing Sjögren’s syndrome A (SSA), SSB, calpastatin and alpha-fodrin in primary Sjögren’s syndrome. Clin Exp Immunol. 2007 May;148(2):281-7.
20. Porola P, Straub RH, Virkki LM, et al. Failure of oral DHEA treatment to increase local salivary androgen outputs of female patients with Sjogren’s syndrome. Scand J Rheumatol. 2011;40(5):387-90.
21. Virkki LM, Porola P, Forsblad-d’Elia H, et al. Dehydroepiandrosteroine (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjogren’s syndrome. Arthritis Care Res (Hoboken). 2010 Jan 15;62(1):118-24.
22. Kim TH, Kang JW, Kim KH, et al. Acupuncture for the Treatment of Dry Eye: A Multicenter Randomised Controlled Trial with Active Comparison Intervention (Artificial Teardrops). PLoS ONE. 7(5):e3668.
23. Shin MS, Kim JI, Lee MS, et al. Acupuncture for treating dry eye: a randomized placebo-controlled trial. Acta Ophthalmol. 2010;88:e328-33.
24. Oke SL, Tracey KJ. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann N Y Acad Sci. 2009 Aug;1172:172-180.


