In the 1966 movie “Fantastic Voyage,” an undercover scientist discovers how to minimize objects including people to the size of cells. But an attempt to assassinate the scientist leaves him comatose with a blood clot in his brain. In order to save him, a submarine is shrunken to 1μm in length, with a similarly minimized crew, and is injected into his blood stream. Ultimately the mission is successful in removing the clot and saving his life.2
When this movie was released in 1966, these concepts seemed truly fantastic, but only a few decades later, today’s nanotechnology has made at least some of these ideas a reality.
What is Nanotechnology?
Nanotechnology is the creation and use of material and devices on the same scale as molecules and intracellular structures, typically less than 100nm in size.3 To put that into perspective, a human hair is 10,000nm to 20,000nm in diameter, and an erythrocyte is 7,000nm wide.3,4
“Nano” is derived from the Greek prefix that means dwarf.5 A nanometer is the amount a man’s beard grows in the time it takes to lift his razor to his face.5 Materials that are considered stable in our everyday world may display very unusual characteristics at nanometric levels; aluminum, for example, may explode spontaneously when its particle size reaches 20nm to 30nm.1
Nanotechnology exists in nature. A strand of DNA is 2nm in diameter, and most proteins are around 10nm size. Naturally occurring nanomotors include kinesin and actin, both essential to muscle movement.5 Research and the eventual application of nanotechnology is shared among many areas of science. In health care, the interest in nanotechnology is focused on its use as diagnostic and therapeutic agents that vastly enhance our ability to detect and treat a broad variety of conditions.5
Nanotechnology and Health Care
A good example of the combined diagnostic and therapeutic application of nanotechnology can be seen in the work of researchers at Rice University’s department of bioengineering in Houston. Naomi Halas, Ph.D., D.Sc., and Jennifer West, Ph.D., originally developed nanoshells, which consist of a silica core (essentially glass) surrounded by an ultrathin layer of gold.5,6 The color of the nanoshell is determined by the size of its core and shell. Most nanoshells are about 100nm in size. Drs. Halsa and West, and colleagues, have investigated their use for detecting and treating tumors. Their research suggests that nanoshells gain access to and accumulate in tumors because of the leakiness of newly formed blood vessels.6
 |
| Manipulating the core and shell thicknesses of gold nanoshells can create a broad range of colors spanning the visible and near infrared spectrum. |
There are several other potential health care applications for nanotechnology including cell repair by manipulation of molecules on an individual basis, reversal of aging changes, removal of plaques and other changes related to cardiovascular disease, repair of bone and neural tissue, gene therapy, assisting stem cells to repair damaged tissue, using nanocomposite contact lenses to monitor blood glucose, and using nanodevices and lasers to perform very focalized surgery.8,9
Nanotechnology and the Eye
The eye is uniquely suitable as a target for nanotechnology. It is a small organ easily accessed due to its exposed position. Nanomaterials have a high surface area to volume ratio. This attribute can be beneficial in reducing or eliminating reactive species, which are established as a cause of cataracts and other ocular disease.10 Specifically, cerium oxide (CeO2) nanoparticles— i.e., nanoceria particles—with a diameter of 5nm have a large surface area to volume ratio and can scavenge reactive oxygen intermediates effectively. Their configuration allows nanoceria particles to regenerate their function as scavengers of radicals without repetitive dosing.11
Junping Chen, M.D., Ph.D., and colleagues have demonstrated that intravitreal injection with nanoceria particles prevents light damage in rodents.11 Future application of this technology may be used to treat other ocular conditions related to oxidative damage, such as macular degeneration and diabetic retinopathy.11
Fluctuation of intraocular pressure (IOP) is characteristic of glaucoma. One issue in managing glaucoma is the measurement of IOP over time, (i.e., at various intervals during the day).12,13 The ability to continuously monitor intraocular pressure would be a valuable asset. Matteo Leonardi, M.D., and colleagues in Switzerland have developed a disposable contact lens with a sensor embedded in it—this contact lens sensor (CLS) allows measurement of the corneal curvature produced by changes in IOP.14
The sensor is a platinum-titanium strain gauge with a total thickness of 175nm.14 The researchers cannulated a pig’s eye, applied a CLS to the cornea and changed IOP in 1mm increments. Their study showed that the CLS was capable of measuring IOP to within 0.2mm Hg (95% confidence interval) of the values obtained with a pressure sensor attached to the eye.
German researchers Christoph Faschinger, M.D., and Georg Mossböck, M.D., have successfully used this system to monitor 11 patients’ IOP’s for up to 24 hours. They recorded pressure profiles that were flat, fluctuating or that had spikes.15
Nanotechnology and Drug Delivery
Researchers are exploring nanotechnology as a means of drug delivery—not only for systemic medications, but also for ocular applications. Many of the conditions that affect the eye are treatable through the ocular surface or vasculature. Conventional delivery systems are not always optimal because of tear dynamics, dilution due to reflexive tearing, relative corneal impermeability to many medications and the influence of ocular surface health on drug absorption. The cornea contains both lipophilic and hydrophilic structures and therefore presents a barrier to many topical medications—typically less than 5% of instilled drug penetrates the cornea.16
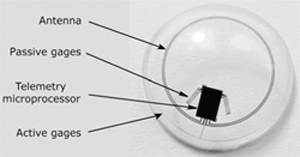 |
| The Swiss-designed prototype contact lens sensor contains
strain gauges that measure corneal curvature changes caused by intraocular pressure variations. A microprocessor and an antenna integrated into the soft contact facilitate wireless powering and communication. |
The composition of the carrier for nanodrugs must be biodegradable, transparent and comfortable. Various biodegradable artificial polymers and natural polymers (e.g., chitosan, gelatin, albumin and sodium alginate) show promise for drug delivery. Piedad Calvo and colleagues investigated bioavailability of the indomethacin, a non-steroidal, anti-inflammatory drug encapsulated in nanoparticles, compared to conventional solution. They found that the in vitro corneal penetration of the encapsulatedindomethacin was more than three-fold that of the commercial eye drops.17
• Liposomes: Liposomes are vesicles consisting of one or more layers of phospholipids—the same base material found in cell membranes that typically contain a core of an aqueous substance. Liposomes can be positive, negative or neutral depending on the chemical composition and core. Their advantages include biocompatibility, bioavailability, low toxicity and the potential to accommodate both hydrophilic and lipophilic drugs.17 Topical dexamethasone poorly penetrates the intact ocular surface. So J. Al-Muhammad and colleagues formulated dexamethasone in liposomes and evaluated concentrations in anterior segments of rabbits. They found high levels of the drug in conjunctiva, suggesting that liposomes create a drug reservoir that enhances ocular penetration.18
• Nanoparticles: Nanoparticles are colloidal systems that have several advantages over conventional delivery. They improve efficiency of delivery, prolong delivery and are particularly useful in chronic diseases such as glaucoma, retinal edema, uveitis and ocular neovascularization. Molecules such as growth factors can be delivered via nanoparticles. In rodent models of glaucoma, nanoparticles loaded with glial-derived neurotrophic factor provided sustained protection of ganglion cells.4
• Dendrimers: Dendrimers are highly branched nanopolymers that have been used to deliver gatifloxacin, pilocarpine and vascular endothelial growth factor (VEGF) inhibitors. Dendrimeric polyguanidilyated translocators (DPT) are nano-sized structures that are used to efficiently translocate molecules across barriers such as cell membranes. Use of DPT produced a four-fold increase solubility and delivery of gatifloxacin to the posterior segment of rabbit eyes and produced a significant increase in tissue concentrations in the conjunctiva and cornea.4 Dendrimers have been formulated as adhesive agents and used by researchers in Boston to do corneal repair and other ocular therapeutic applications requiring an adhesive. These products hold great promise as an alternative to sutures in penetrating ophthalmic injuries.19
New Surgical Techniques
One of the most fascinating techology is axon repair at the subcellular scale. Severed axons in the central nervous system (CNS) do not regenerate from their axon stump, at least in part because of inhibition by the surrounding environment. Adult CNS neurons cannot regenerate. Severed peripheral nervous system (PNS) axons can regenerate, but must do so through the endoneural sheaths that extend to the original target location. Current reconstructive surgery of the PNS is aimed at precisely directing axons into the neural sheaths, a goal that is often not achieved.20
With the advent of nanotechnology, CNS surgery is changing. Currently, CNS uses “nerve constructs,” artificially manufactured tissues that bridge the gap between the proximal and distal stumps nerve. One approach is to use nanoscale materials to join nerves. Nanoknitting uses 5nm peptides to support the nerve and minimize the inhibitory factors present. This technique has been successfully performed on hamster retinal axons. Similar studies have been conducted on spinal cord injuries in rats.20
 |
| The commercial version of the contact lens sensor, the Triggerfish (Sensimed AG, Lausanne, Switzerland) received the CE designation in 2009, and differs slightly in
appearance from the earlier prototype. |
These instruments require an amazingly precise means of manipulating the material to be severed; diaelectrophoresis (DEP) is one means of achieving such precision. DEP moves a polarizable object in a nonhomogeneous electrical field by exerting forces that cause it to move toward one side of the electrical field. The magnitude of movement is dictated by DEP and is directly related to strength of the applied electrical field, the AC frequency, the size and shape of the object and its dielectric properties. DEP has been successfully utilized to manipulate bacteria cells and nerve axons.20
Once axons have been aligned and damaged tissue removed, it is necessary to fuse the two segments. One method, electrofusion, uses electrical pulses to create a transient breakdown of cell membranes and the formation of pores which, if the pores are formed in regions of adjacent axons, will cause fusion between the two cells. Other methods, including chemical fusion and laser-trapping fusion, have been evaluated for axon repair. 20
The use of nanotechnology has the potential to revolutionize the way we deliver and receive health care. This is particularly true for the eye and the nervous system. Some of the technologies currently in trials were only dreams less than a decade ago. Both the profession and our patients will benefit as advances in new mediation delivery systems and diagnostic systems continue to emerge. However, this is not without possible risks and disadvantages— as with any technology, there may be unanticipated side effects and complications.
It is highly unlikely that any of us will be miniaturized to explore our patient’s eyes through a nanosubmarine, but nanotechnology can offer us the ability to see and accomplish equally amazing feats.
William Townsend, O.D., is a graduate of the University of Houston College of Optometry and practices in a multi-location setting. He is Distinguished Visiting Clinician in Residence, an adjunct professor at the University of Houston College of Optometry and the current president of the Ocular Surface Society of Optometry.
1. Kahn J. Nano’s big future. National Geographic. 2006 June:98-119.
2. Fantastic Voyage. Wikipedia. Available at: en.wikipedia.org/wiki/Fantastic_Voyage (Accessed September 2011).
3. Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanotechnology in ophthalmology. Can J Ophthalmol. 2010 Oct;45(5):457-76.
4. Burke P. Nanotechnology. 2005 Spring. Graduate class taught at University of California, Irvine.
5. Glassman G. Working with nanoshells. NOVA science NOW. 2005 Apr. Available at: www.pbs.org/wgbh/nova/body/halasnanoshell. html (accessed October 2011).
6. Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonicsbased imaging and therapy of cancer. Technol Cancer Res Treat. 2004 Feb;3(1):33-40.
7. Gobin AM, Lee MH, Halas NJ, et al. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007 Jul;7(7):1929-34.
8. 25 ways nanotechnology is revolutionizing medicine. Future-Medica. 2010 Jan 19. Available at: mritechnicianschools.net/2010/25-ways-nanotechnology-is-revolutionalizing-medicine (accessed October 2011).
9. Babizhayev MA. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract disease. Cell Biochem Funct. 2011 Apr;29(3):183-206.
10. Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature Nanotechnology. 2006 Nov;1(2):142-50.
11. Pajic B, Pajic-Eggspuchler B, Haefliger I. Continuous IOP fluctuation recording in normal tension glaucoma patients. Curr Eye Res. 2011 Oct 6.
Additional references available at www.reviewcontactlenses.com.


