With reusable contact lenses, the storage case can be a significant source of microbial contamination and potential infection.1 Understanding the complexity of lens case development, chemistry and microbial contamination is vital to keeping lens wearers safe and healthy.
FDA Regulations
The Food and Drug Administration (FDA) gives clearance for medical devices to be sold in the United States based on device categories. The FDA has three regulatory classifications of medical devices—Class I, Class II and Class III—assigned by the risk the medical device presents to the patient and the level of regulatory control needed to legally market the device.
As the classification level increases, the risk to the patient and FDA regulatory control increases. Accessories to medical devices are considered the same classification as the medical device (i.e., lens cases and contact lenses). Class I devices present the least amount of potential user harm and have the least amount of regulatory control, while class III devices present significant risk of illness or injury to the patient and are heavily regulated.
Class II: Contact Lens Cases
Contact lens cases are class II devices. Class II medical devices have potential user risk; however, there are existing methods/standards/guidance documents available to provide assurances of safety and effectiveness. Class II devices typically require pre-market notification by submission and FDA review of a 510(k) clearance to market submission.2 Class II devices are required to have special labeling, mandatory performance standards and postmarket surveillance.
As a class II device, contact lens cases must go through the 510(k) process to show substantial equivalence to a currently marketed lens cases. The FDA does not require microbial testing of lens cases unless the manufacturer claims the case has antimicrobial properties. Clinical testing of the case alone is not required. Heat testing is needed only if the case is to be used specifically for heat disinfection. Unless the Material Safety Data Sheet from the manufacturer is supplied, the lens case plastic requires toxicity testing.
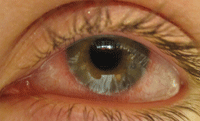
Contact lens-associated red eye.
The Case Design
The well size determines how much biocidal storage solution surrounds the dormant contact lens. The International Organization for Standardization (ISO) 14729 is the “microbiological requirements and test methods for products and regimes for hygienic management of contact lenses.”3
A stand-alone test is a measure of the innate anti-microbial activity of the contact lens disinfectant to kill an appropriate level of microorganisms within the allotted period of time. Each solution is challenged with five different microorganisms. If the product meets the requirements of the test, the product can be labeled a contact lens disinfectant. If the product does not meet this standard, the contact lens must go through mechanical cleansing (a regimen) to meet the minimum disinfection requirements.
Therefore, the amount of solution needed for the lenses is in proportion to the efficacy of the disinfectant, how dirty the lens is and how long it will be stored. For patients, this means for a stand-alone (no-rub) product, the well size of the associated lens case is important and should be completely filled in order to achieve the FDA standards.3
Even if the product meets ISO standards, the lens storage container material may affect efficacy of the product. Biocide is constantly being absorbed and adsorbed by the lens case. When a disinfectant adheres to the container, it leaves very little in the solution to interact with the contact lens, leaving the lens vulnerable to contamination. Manufacturers generally design a special lens container to maximize the disinfection for their particular solution.4 Using alternate cases for a given solution may affect biocidal patterns. The topology of the lens case well grooves also affects biofilm formation and ease of contaminant removal.5,6
What Grows Inside?
Lens cases are contaminated with bacteria, fungi or protozoa about 19% to 81% of the time.1 Contamination can come from failure to clean and store cases properly, dirty fingers, climate, “topping off,” tap water and variable storage times.
The most common pathologic organisms found in lens cases include Pseudomonas, Serratia, Staphylococcus, Acanthamoeba and Fusarium. Contact lens associated red eye has been associated with Haemophilus influenzae, Acinetobacter sp., Pseudomonas aeruginosa, Aeromonas hydrophila, Serratia liquefaciens, Serratia marcescens and Pseudomonas putida. Infiltrative keratitis and CLPU have been associated with Staphylococcus aureus, Streptococcus pneumoniae, Abiotrophia defectiva and Acinetobacter sp.1
Amoebic contamination rates of lens cases ranges from zero to 20%. Acanthamoeba, both trophozoites and cysts, can rapidly adhere to the lens case after exposure to the organisms. There is evidence that adherence occurs at greater frequency in used cases than in new cases.6 This is probably due to surface roughness and residual water droplets.
In theory, these microorganisms should be killed when exposed to contact lens solution. In reality, microorganisms form biofilms, which protect them from biocidal destruction. Biofilms grow through a combination of cell division and recruitment, starting with the attachment of free-floating (planktonic) microorganisms to the lens case surface. Other planktonic cells can then attach to the adhered bacteria. Eventually there are many layers of microorganisms living within a matrix of extracellular DNA, proteins and polysaccharides.
A biofilm can be formed by a single microorganism species, but more often we find biofilms that consist of many species of bacteria, fungi, algae and protozoa. Biofilms have increased resistance to detergents and antibiotics, since the surrounding matrix and the outer layer of cells protect the inner colonies. A biofilm also produces high levels of antibiotic degrading enzymes. Repeated use of antimicrobial agents on biofilms can cause bacteria within the biofilm to develop an increased resistance to biocides. Data suggests that microbial keratitis events involve biofilm-forming organisms; therefore, removing the biofilm from the contact lens case is an important step in lens care compliance.7
Caring for Your Case
Numerous studies have now shown that mechanically wiping the contact lens case dry after lens removal will mechanically disrupt the biofilm and will significantly reduce the lens case bioburden.6,8,9 Air drying the lens case upside down further reduces contamination.
Silver impregnated lens cases have less biofilm formation than polypropylene lens cases. Silver has low toxicity and its multiple sites of action provide a low potential for developing bacterial resistance. The silver ions are only released when moisture comes into contact with the cases. Therefore, to continue the anti-microbial activity when not storing lenses, silver impregnated cases should be rinsed with solution and stored with the cap on—as opposed to conventional containers which should be stored with the cap off.10
Biofilm, topology, material and care of lens cases can and do affect our patients contact lens safety and wearing experience. We must take the time to understand and properly educate our patients on effective lens care.
1. Szczotka-Flynn L, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens. 2010 Mar;36(2):116-29.
2. United States Food & Drug Administration. Available at:
www.fda.gov (accessed February 2012).
3. Ophthalmic optics—Contact lens care products—Microbiological requirements and test methods for products and regimens for hygienic management of contact lenses. ISO 14729. 2001.
4. Van Duzee B, Schlech B. The activity of multi-purpose solution against Acanthamoeba. Optician. 1999;217:34-5.
5. Wu YT, Zhu H, Willcox M, Stapleton F. The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. IOVS. 2011 Jul;52(8):5287-92.
6. Boost M, Shi GS, Cho P. Adherence of Acanthamoeba to lens cases and effects of drying on survival. Optom Vis Sci. 2011 Jun;88(6):703-7.
7. Tam C, Mun JJ, Evans DJ, Fleiszig SM. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest Ophthalmol Vis Sci. 2010 Jun;51(6):3100-6.
8. Abengózar-Vela A, Pinto FJ, González-Méijome JM, et al. Contact lens case cleaning procedures affect storage solution pH and osmolality. Optom Vis Sci. 2011 Dec;88(12):1414-21.
9. Wu YT, Zhu H, Willcox M, Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalm Vis Sci. 2010 Dec;51(12): 6329-33.
10. Dantam J, Zhu H, Stapleton F. Biocidal efficacy of silver-impregnated contact lens storage cases in vitro. Invest Ophthalmol Vis Sci. 2011 Jan;52(1):51-7


