 We became eye care professionals to serve patients in need of visual and ocular rehabilitation. Through clinical experience, we now understand that some of our prescribed treatments can potentially cause some degree of harm due to the preservative contained within the bottle. It is our job as practitioners to determine the risk-to-benefit ratio for each patient and their individual needs before writing a prescription.
We became eye care professionals to serve patients in need of visual and ocular rehabilitation. Through clinical experience, we now understand that some of our prescribed treatments can potentially cause some degree of harm due to the preservative contained within the bottle. It is our job as practitioners to determine the risk-to-benefit ratio for each patient and their individual needs before writing a prescription.
Preservative agents are essential in minimizing the risk of bacterial and non-bacterial infections in multiple-use ophthalmic bottles. However, some preservatives are associated with ocular surface changes and symptoms including tear film instability, inflammation and subjective discomfort.1 The most frequently implicated offender is benzalkonium chloride (BAK)—a quaternary ammonium detergent preservative shown to reside in ocular tissues for up to seven days, which allows for greater medicine penetration but also causes cellular damage.2 BAK is degraded to hydrogen peroxide, which is associated with considerable surface irritations and increased localized inflammation.3
Preservative sensitivity can become a significant issue in the use of chronic medications such as those used to treat glaucoma and dry eye. Typically, patients on these therapies are older and already suffer from ocular dryness from decreased tear production and blink rate. In a multicenter cross-sectional epidemiological survey of 9,658 patients in four European countries, Nele Jaenen, M.D., and colleagues demonstrated that those who used a preserved glaucoma medication reported a statistically significant increase in ocular symptoms compared to patients using preservative-free medication.4 The most common symptoms were pain or discomfort upon instillation (47.6% vs. 18.5%) and stinging or burning (47.5% vs. 19.6%). Objectively, corneal and conjunctival signs—including blepharitis, eczema, hyperemia, follicles and superficial punctuate keratitis—were two- to three-times more prevalent in the group using preserved drops. The duration of glaucoma treatment is implicated in reduced tear secretion—measured by Schirmer 1 and Jones tests—as well as conjunctival changes.5 David Ammar, Ph.D., and colleagues noted that glaucoma medications containing sofZia—such as Travatan Z (travopost, Alcon)—showed less cytotoxicity to epithelial and conjunctival cells than assays containing BAK or polyquad derivatives.6
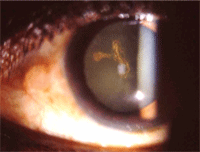
Dendritic HSV ulcer.
Bottled, preserved artificial tears, while prescribed to treat dry eyes, can coincidentally exacerbate the problem they were intended to treat by inducing toxic keratoconjunctivitis if dosed inappropriately.7 Dry eye patients were found to have corneal epithelial permeability that was 2.7-times that of a control group without ocular surface disease. Patients treated with unpreserved polyvinyl pyrrolidone 2% had a 37% decrease in permeability, while those treated with polyvinyl pyrrolidone 2% preserved with benzalkonium chloride 0.005% showed a 21% increase in permeability. For patients requiring more frequent dosing, or those with fragile epithelia, it is advisable to switch to the many available non-preserved artificial tear preparations.8
Drugs used on a short-term basis—antibiotics, anti-inflammatories and antivirals—can also cause iatrogenic toxicity. In a comparison of five antibiotics and two non-steroidal medications, the anti-inflammatory medications were more often associated with epithelial cell loss and postoperative keratitis than the antibiotics.9 In a comparison among ocular preservatives and their kill rates on human conjunctival and corneal epithelial cells, thimerosal was found to be the most destructive.10 In addition to epithelial cell toxicity, cicatricial changes of the conjunctiva and limbus have been associated. It is peculiar to note that thimerosal is still found among topical ocular solutions, particularly Viroptic (trifluridine, Monarch Pharmaceuticals). Thus, it seems wise to exercise caution when prescribing the medication and look for ocular surface changes not consistent with the spread of the dendrite. Some practitioners have turned to Zirgan (ganciclovir, Bausch + Lomb). Although Zirgan is BAK preserved, it is dosed less often and comes in gel form so it is more comfortable than Viroptic. Others have advocated the treatment of epithelial dendritic keratitis with strictly oral agents, such as acyclovir. While some data supports the drug’s equivalency, it is not FDA approved for this purpose and an increased systemic involvement has been reported.11
We need to remember that an eyedrop that is uncomfortable is an eyedrop that the patient will not be compliant with, but at the same time, it is naïve to assume that the solution is to only prescribe non-preserved preparations of all the drops our patients require. This is especially true when you take into consideration that single dose units are more costly, patients with dexterity issues may have difficulty opening the vial and corneal abrasions can occur from the resultant sharp edge of the container. Additionally, not all preservative-free drops are commercially available, or able to be specially compounded. That said, as we reach for our prescription pad, we should be cognizant of the fact that there may be some associated risk for the patient. For instance, since many regularly prescribed eye drops contain BAK—and its collateral damage is cumulative over time—we should take care to not use multiple BAK preserved medications concurrently. We should strive to keep manageable risks at a minimum whenever possible by choosing an alternative drug with a different preservative or to opt for a non-preserved version of our drug of choice when possible.
1. Wilson FM. Adverse external ocular effects of topical ophthalmic medications. Surv Ophthalmol. 1979;24:57-88.
2. Burstein NL. The effects of topical drugs and preservatives on the tears and corneal epithelium in dry eye. Trans Ophthalmol Soc. 1985;104(Pt4):402-9.
3. Epstein SP, Chen D, Asbell P. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009 Oct;25(5):415-24.
4. Jaenen N, Baudouin C, Pouliquen P, et al. Ocular signs and symptoms with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007 May-Jun;17: 341-9.
5. Nuzzi R, Finazzo C, Cerruti A. Adverse effects of topical antiglaucoma medications on the conjunctiva and lachrymal response. Int Ophthalmol. 1998;22(1):31-5.
6. Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human epithelial cells. Adv Ther. 2010 Nov;27(11):837-45.
7. Gobbels M, Spitznas M. Corneal epithelial permeability of dry eyes before and after treatment with artificial tears. Ophthalmology. 1992 Jun;99(6):873-8.
8. Geerling G, Daniels JT, Dart JKG, et al. Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001 Apr;42:948-56.
9. Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009 Apr;25(2):113-9.
10. Collum LM, McGettrick P, Akhtar J, et al. Oral acyclovir (Zovirax) in herpes simplex dendritic corneal ulceration. Br J Ophthalmol. 1986 Jun;70(6):435-8.


