 |
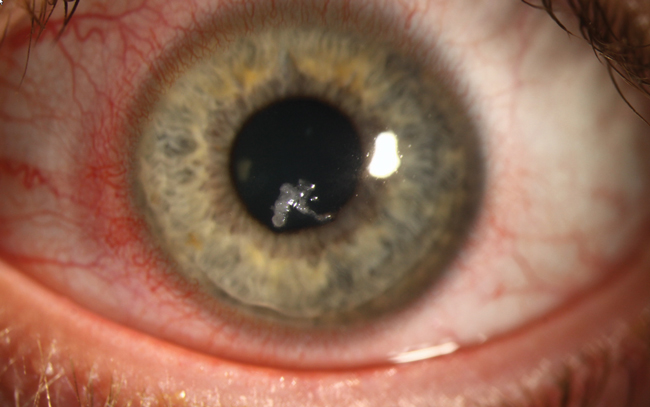
A 34-year-old male who had undergone LASIK in 2003 experienced progressive ectasia and was referred for corneal collagen crosslinking (CXL). Two weeks s/p, he presented with entrapped mucus in the epithelium. Superficial keratectomy was performed to clear the cornea, and a bandage contact lens was placed to alleviate pain and encourage healing. He was prescribed levofloxacin QID, prednisolone acetate 1% TID with taper and ibuprofen 800mg Q8hr PRN for discomfort. One week later, the patient presented with a central corneal ulcer, depicted here.
Pinpointing causality in the chain of events is difficult in such cases. This patient underwent the standard FDA-approved ‘epi-off’ CXL procedure, had an epithelial complication that required superficial keratectomy and, despite antibiotic coverage, developed ulceration.
In crosslinking, as in all surgical procedures, there may be unforeseen adverse events (AEs) postoperatively. The FDA trials of Photrexa and the KXL system (Avedro) reported corneal epithelial defect in 28% and keratitis in 3% of ectasia patients.1 Other notable AEs in this group included corneal opacity/haze (71%), striae (9%), dry eye (14%), pain (26%), punctate keratitis (20%), photophobia (19%), reduced acuity (11%) and blurred vision (17%)—all expected sequelae after epithelial corneal debridement that occurred at a higher incidence than in controls.1
Epithelial defect, striae, punctate keratitis, photophobia, dry eye, pain and decreased acuity took up to six months to resolve, and corneal opacity/haze took up to 12 months.1 In 6% of ectasia patients, corneal opacity remained at 12 months.1 Looking at the study population as a whole, most other AEs were mild and resolved during the first month.1
Although risk of vision-impairing complications with CXL is low, clinicians should remain vigilant, particularly during epithelial regrowth when the cornea is most vulnerable to infection. Our patient was started on a broad-spectrum antibiotic while we await culture results.
1. Highlights of prescribing information, Photrexa Viscous and Photrexa, for use with the KXL System, Avedro. Revised Sept. 2017.
 |


