Imagine a world where the physical characteristics of a material change as it becomes smaller, or where submicroscopic surgical instruments can be used to repair damaged nerves.1,2 This may sound like a scene out of a science fiction movie, but it is our reality today. More so, this technology provides an excellent opportunity to diagnose and treat disease at the molecular level. What are we talking about? It is the world of nanoscience, nanotechnology and nanomedicine.3
A Nano Approach
Let’s start by defining the key players:
- Nanoscience refers to the “fundamental study of phenomena and the manipulation of matter at the atomic, molecular and supramolecular level, where properties differ significantly from those at a larger scale.”4
- Nanotechnology denotes “the design, characterization, production and application of structures, devices and systems that have novel physical, chemical and biological properties by controlling shape and size at the nanometer scale.”4
- Nanomedicine encompasses a broad range of technologies. It is defined as, “the science and technology of diagnosing, treating and preventing disease and traumatic injury; of relieving pain and of preserving and improving human health; and using nanoscale structured materials, biotechnology, genetic engineering, and eventually complex machine systems and nanorobots.”5
Derived from the Greek word nano, or dwarf, a nanometer is one billionth (10-9) of a meter.6 Conceptualizing something that minute can be challenging, so contemplate this: A nanometer is proportional to a meter in the same way that a marble is comparative to the earth.6 To further put that into perspective, the diameter of a double strand of DNA is around 2nm.6 Using nanotechnology, we can now manufacture particles no larger than a viral particle or bacterium.
Nanoparticles typically are defined as clusters of atoms that range in size from 1nm to 100nm (some objects up to 200nm may still fall under this definition).3,6 The properties of a material in nanoscale may differ dramatically from the properties it exhibits as larger particles. For example, in both the “conventional” scale (e.g., a can) or in the “micro” scale (e.g., powder), aluminum possesses the same basic qualities. However, as a nanoparticle aluminum can spontaneously burst into flames.1
Implications for the Eye
Nanotechology offers great promise in health care. The particles are roughly the same size––or, in some cases, smaller than––the infectious agents that cause disease. For example, a virus is roughly 100nm in diameter, while bacteria are about 10 times as large. Nanoparticles are also similar in size to basic physiologic molecules found in nature: A glucose molecule is approximately 1nm in diameter and an antibody is roughly 10nm in size.3
The eye, in particular, may be a very viable site for nanotherapy. The lipophilic corneal epithelium, hydrophilic stroma of the cornea and sclera, conjunctival lymphatics, and blood-eye barrier often make it difficult for molecules to be absorbed into the eye.7 Topical administration is usually the preferred means of delivering a medication to the eye but, because of the factors previously mentioned, it may be an inconsistent or inefficient means of conveying molecules into the eye. Typically, less than 5% of a topically applied dose reaches the anterior ocular tissues.7 Incorporating a therapeutic molecule into a nanoparticle may allow better targeting of specific tissues or organs using a significantly lower concentration of drug.
Medications successfully formulated from nanoparticles theoretically would have the potential to deliver adequate intraocular concentrations of a drug to the anterior and posterior eye at lower surface concentrations.7 This is partially due their small size and the type of structures they form.
Drug Delivery
Our understanding of glaucoma has increased exponentially over the past two decades. Still, the reduction of intraocular pressure continues to be the primary method of treating this condition. Unfortunately, current therapy is not free of complications or side effects, which can negatively impact a patient’s quality of life.8 Treatment itself also may be unsuccessful, especially when you consider that patients may forget to take their medications and/or not be able to pay for the costly doses. In addition, preservatives in glaucoma medications can negatively affect the ocular surface to the point of surgical intervention.9
Consider the benefits of incorporating nanomolecules into ocular tension-lowering medications. Jayaganesh Natarajan, PhD, and colleagues fabricated latanoprost-loaded egg-phosphatidylcholine (EggPC) liposomes, each with an approximate diameter of 109nm. Following a single subconjunctival injection of the liposome formulation, they monitored the clinical status of the eyes and recorded the intraocular pressure. They noted sustained release of latanoprost, with 60% of the drug discharged after 14 days. The intraocular pressure-lowering effect was still maintained after 90 days, and there was a significantly greater mean IOP reduction in the eyes treated with subconjunctival injection of the liposome formulation vs. the eyes treated daily with topical latanoprost solution.10
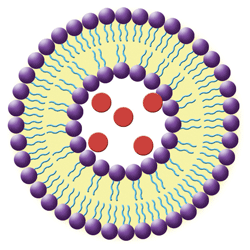
1. In this simple liposome structure, hydrophilic heads (purple spheres) orient to the outside surfaces and the lipophilic tails (blue lines) orient toward the inside. Hydrophilic medications (red spheres) are trapped in the aqueous-filled central cavity.
• Liposomes are nanoscale spherical vesicles that can be produced from natural phospholipids and cholesterol (see figure 1). Their structure forms when phospholipids are combined with water; the immediate result is a bilayered sphere. One end of each molecule is water soluble, while the opposite end is water insoluble. This configuration allows for delivery of both lipophilic drug molecules incorporated into the bilayer and water-soluble drugs that are trapped inside the liposomal cavity.
• Micelles are lipid molecules arranged in a spherical form in aqueous solutions (see figure 2).11 Their configuration resembles that of liposomes and results from the amphipathic nature of fatty acids (e.g., they contain both a hydrophobic and hydrophilic [polar] end groups). In water, the polar end faces toward the outer surface of the micelle.11 Micelles offer distinct advantages over conventional delivery because of their small size, reduced toxicity, ability to solubilize drugs and targetability.12
Several studies have evaluated the feasibility of micelles as carriers for ophthalmic medications. When applied topically onto the eye, cyclosporin A (CsA) physically entrapped into micelles displayed a prolonged residence time.13 Topical ketorolac physically entrapped into micelles was compared to the administration of the conventional aqueous preparation; corneal penetration of the micelle preparation was significantly higher than the conventional aqueous suspension and demonstrated higher inflammatory activity.14 These studies suggest that micelles also may prove to be an excellent alternative to conventional ophthalmic preparations.
Severe uveitis is a potentially blinding disease that is often treated with triamcinolone acetonide (TA). Because TA is a water-insoluble corticosteroid, it is typically delivered by subtenon, subconjunctival or intravitreal injection.15 Intravitreal steroid injection may cause complications, including cataract formation, retinal detachment, hemorrhage and intraocular pressure elevation. In a study using a rabbit model of uveitis, María Rodríguez-Blanco and colleagues compared intravitreal TA suspension to topical TA in nanoparticles (TA-NP) and topical prednisolone acetate (PA) suspension.16
There was no statistical significance in the anti-inflammatory effect between the intravitreal TA and the topical TA-NP, and both were significantly more effective than topical PA in reducing inflammation.15 While this is an animal-based study, it demonstrates that topical NP provides a pharmaceutical benefit comparable to intravitreal injections of the conventional form of the drug.
Antimicrobial Efficiency
The recent increase in antibiotic-resistant bacteria is an issue of great concern to health care providers. This phenomenon has been attributed to several factors, including overprescribing of antibiotics.17 One plausible means of circumventing this problem is through the use of nanoparticle carriers of antibiotics. By modifying materials at the atomic level, nano-sized organic and inorganic particles can be generated for eventual use in health care.18
The capability of nanoparticles to kill bacteria largely is a simple matter of size. Bacterial cell size is in the micrometric range (500nm to 5000nm). Bacterial outer cellular membranes have pores in the nanometer range (5nm to 50nm). Because nanoparticles can be smaller in size than bacterial pores, they can actually cross cell membranes. One example of this is the use of nano-sized molecules containing metal oxides.19
Ameer Azam, PhD, and colleagues evaluated the antimicrobial efficacy of nano-sized particles of zinc oxide 18nm (ZnO), copper oxide 22nm (CuO) and ferrous oxide 28nm (Fe2O3). The nanoparticles were synthesized by gel-combustion method and their antibacterial activities were tested against two gram-positive bacterial strains (S. aureus and Bacillus subtilis) and two gram-negative bacterial strains (Pseudomonas aeruginosa and E. coli).19 The results revealed that ZnO and CuO nanoparticles had excellent antibacterial activity against both gram-positive and gram-negative bacteria. The evidence generally indicates that the smaller the size of the nanoparticle, the more active it is against bacteria. For example, ZnO was 75% more effective than Fe2O3, and 28% more effective than CuO against E. coli.19
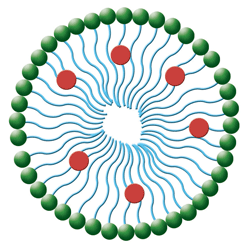
2. In this simple micelle structure, hydrophilic heads (green spheres) orient to the outside surface and the lipophilic tails (blue lines) orient toward the inside. The medication molecules (red spheres) attach to the lipophilic tails.
Justin T. Seil, PhD, and Thomas J. Webster, PhD, confirmed the significant antibacterial effect of ZnO. They reported that, after just eight hours of exposure to ZnO nanoparticles, there was in excess of a four-log reduction in Staphylococcus aureus. When they added ultrasound to the nanoparticle regimen, they noted an additional 76% reduction in the number of viable colony-forming units.20
The size and unique attributes of the eye make it an excellent subject for nanotechnology. The applications discussed in this article represent just a small fraction of the research and technology already completed and under development.
We are learning that the potential applications of nanotechnology within eye care are almost limitless and nanotechnology may eradicate some of the conditions and illnesses that steal our vision, shorten our lives or reduce our quality of life.
William Townsend, OD, is a graduate of the University of Houston College of Optometry and practices in a multi-location setting. He is Distinguished Visiting Clinician in Residence, an adjunct professor at the University of Houston College of Optometry and the current president of the Ocular Surface Society of Optometry.
1. Kahn J. Nano’s big future. National Geographic. 2006 June:98-119.
2. Freitas RA Jr. Nanotechnology, nanomedicine and nanosurgery. Int J Surg. 2005;3(4):243-6.
3. Filipponi L, Sutherland D. NANOYOU: Teachers training kit in nanoscience and nanotechnology. 2010 Jan. Available at:
http://nanoyou.eu/attachments/052_NANOYOU%20Overview%20of%20training%20kit_%20for%20website.pdf. Accessed April 2013.
4. Mette Ebbesen M, Jensen TG. Nanomedicine: techniques, potentials, and ethical implications. J Biomed Biotechnol. 2006 Nov 30:51516.
5. Patil M, Mehta DS, Guvva S. Future impact of nanotechnology on medicine and dentistry. J Indian Soc Periodontol. 2008 May-Aug;12(2):34-40.
6. Photocatalyst nanotechnology. Green Earth Nano Science Inc. Available at:
www.greenearthnanoscience.com/nanotechnology.php. Accessed April 2013.
7. Vadlapudi AD, Mitra AK. Nanomicelles: an emerging platform for drug deliver to the eye. Ther Deliv. 2013 Jan;4(1):1-3.
8. Nordmann JP, Auzanneau N, Ricard S, Berdeaux G. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003 Dec 10;1:75.
9. Pavici-Astalos J, Lacmanovi-Loncar V, Petric-Vickovi I, et al. Eye drops preservative as the cause of corneal band keratopathy in long-term pilocarpine hydrochloride treatment. Acta Clin Croat. 2012 Mar;51(1):107-11.
10. Natarajan JV, Ang M, Darwitan A, et al. Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomedicine 2012:7;123-131.
11. Structural biochemistry/lipids/micelles. Wikibooks. Available at:
http://en.wikibooks.org/wiki/Structural_Biochemistry/Lipids/Micelles. Accessed April 2013.
12. Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond). 2010 April;5(3):485-505.
13. Di Tommaso C, Torriglia A, Furrer P, et al. Ocular biocompatibility of novel Cyclosporin A formulations based on methoxy poly(ethylene glycol)-hexylsubstituted poly(lactide) micelle carriers. Int J Pharm. 2011 Sep 20;416(2):515-24.
14. Pepic I, Lovri J, Filipovi-Gri J. Polymeric micelles in ocular drug delivery: rationale, strategies and challenges. Chem Biochem Eng Q. 2012;26(4):365-77.
15. Sabzevari V. Polymeric triamcinolone acetonide nanoparticles as a new alternative in the treatment of uveitis: In vitro and in vivo studies. Eur J Pharm Biopharm. 2013 Jan 5.
16. Rodríguez-Blanco M, Marticorena J, Gómez-Ulla F. Triamcinolone acetonide for refractory pseudophakic cystoid macular edema after intravitreal bevacizumab.
Graefes Arch Clin Exp Ophthalmol. 2009 Mar;247(3):427-8.
17. Porco TC, Gao D, Scott JC, et al. When does overuse of antibiotics become a tragedy of the commons? PLoS One. 2012;7(12):e46505.
18. Leary JF. Nanotechnology: what is it and why is small so big? Can J Ophthalmol. 2010 Oct;45(5):449-56.
19. Azam A, Ahmed AS, Oves M, et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012;7:6003-9.
20. Seil JT, Webster TJ. Antibacterial effect of zinc oxide nanoparticles combined with ultrasound. Nanotechnology. 2012 Dec 14;23(49):495101.
21. Ramos-Cabrer P, Campos F. Liposomes and nanotechnology in drug development: focus on neurological targets. Int J Nanomedicine. 2013;8:951-60.
22. Liposomal nanopharmaceuticals. NanoPharmaceuticals. Available at:
www.nanopharmaceuticals.org/Liposomes.html. Accessed April 2013.


