As eye care practitioners, we are fortunate to have an increasingly large bag of tricks to turn to for the management and care of our patients. Yet, if we were to critically analyze our day-to-day practice, we might find that we consistently reach for the same remedies time and time again. In other words, are we truly tailoring the treatment to our individual patient––or are we inadvertently using a more cookie cutter, “one size fits all,” approach?
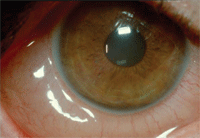
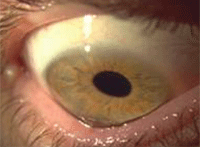
Allergic conjunctivitis.
Between professional meetings and published journal articles, there has been much buzz about the discovery and potential use of biomarkers for different disease states in the last few years. Can a particular disease, or the severity of that disease, be ascertained via blood, tissue or fluid samples—therefore leading to safer, more efficacious and cost-effective treatments, while minimizing unnecessary procedures, costs and toxicity?
The potential applications in medical practice are boundless.
Biomarkers themselves are not a completely new concept. We consistently use several biomarkers to diagnose and monitor several disease states (e.g., cholesterol levels as an indication for the risk of coronary and vascular disease; C-reactive protein for inflammation; viral load for HIV; HbA1C for diabetes). However, the identification of biomarkers in tears may allow for the identification and grading of these conditions, which will ultimately lead to the development of a targeted drug therapy.
Tears are of particular interest because they contain proteins in high concentration; the location of the eye makes them relatively easy to collect through Schirmer strips or micropipettes. In this article, I will review some of the research concerning tear biomarkers and their potential clinical applications for practices—specifically in the areas of dry eye, ocular allergy and keratoconus.
Early Detection: Dry Eye
Clinically speaking, we are often faced with the dilemma where objective signs and subjective symptoms do not correlate, making management decisions challenging at best. Should we treat based on what we see in the slit lamp, or by what the patient tells us? Kelly Nichols, O.D., Ph.D., M.P.H., evaluated 75 patients in a university-based clinic to determine the repeatability and validity of dry eye testing and symptoms in a dry eye population.1 Dr. Nichols found no significant correlation between any individual symptom and individual sign, nor any individual sign and group of symptoms. She also found that a reduction in patient-reported symptoms did not correlate with clinical signs. So, how can a clinician design optimal and effective treatment when faced with such contradictions? Let’s look at the research.
In 2007, the Dry Eye Workshop (DEWS) Report characterized dry eye into two categories: aqueous deficient (with two subcategories—Sjögren’s Syndrome dry eye and non-Sjögren’s Syndrome dry eye) and evaporative.2,3 As clinicians, we recognize that there is often overlap between the two classes. DEWS also emphasized the major roles of increased osmolarity and inflammation in the mechanism of dry eye disease. Hyperosmolarity is known to induce apoptosis, promote inflammatory stress on the ocular surface and reduce the ability of mucin-like molecules to lubricate the ocular surface.4-6 Tear osmolarity has been reported in the literature as being elevated in patients with keratoconjunctivitis sicca, as compared to normal eyes, and to correlate with the severity of the disease state.7,8
Michael Lemp, M.D., and colleagues found that tear film hyperosmolarity was the best marker of dry eye disease severity across normal, mild/moderate and severe categories in a prospective, 10-center study of 299 patients.9 Clinical testing included osmolarity, Schirmer strips, tear film break-up time, corneal and conjunctival staining and meibomian gland assessment. An Ocular Surface Disease Index (OSDI) questionnaire was also employed. The authors utilized the TearLab Osmolarity System, which requires as little as 50nl of tear fluid and can assess osmolarity in less than one minute. The TearLab Osmolarity System has been cleared by the FDA as a 510(k) device.9,10
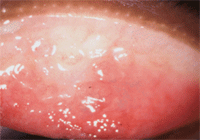
Allergic papillary reaction.
Tear film osmolarity was found to be an excellent biomarker for dry eye severity in a study by Mari Suzuki, M.D., and colleagues.11 They evaluated 19 dry eye patients with a tear osmometer that required just 5µl to 10µl of tear sample and, despite the small sample size, found a significant correlation between hyperosmolarity and dry eye disease severity.11
Hyperosmolarity can identify asymptomatic patients prior to clinically apparent ocular surface damage; repeat osmolarity measurements can help gauge therapeutic efficacy. J. Daniel Nelson, M.D., and R. Linsy Farris, M.D., studied patients with moderately severe dry eye, with a preparation of 0.1% sodium hyaluronate: The average osmolarity of the HA group was significantly reduced from 339mOsms/L to 312mOsms/L (p < 0.05). This change was immediate and steady over the course of the eight-week study, and preceded the reduction of pain and discomfort scoring by several weeks.12 An osmolarity score of either 316mOSms/L or 317mOSms/L has been proposed as a cutoff value for dry eye disease.13,14
These studies demonstrate how tear film osmolarity testing has transitioned from the research lab into the clinical setting with simplified collection of only a small volume of tears and quick in-office assessment—making it an important and relevant biomarker and allowing for more individualized treatment.
What the Research Tells Us
Research in the area of tear film proteomics is a new and exciting avenue that may uncover complex and specific proteins that can differentiate a healthy from a diseased eye.
Kari Green-Church, Ph.D., and colleagues, tested various proteomic approaches. including SELDI and gel electrophoresis from tears collected via Schirmer strips or glass microcapillary tubes and found 97 unique proteins in tears of normal, non-contact lens wearing patients.15 Jingling You, M.D., and his group were able to identify over 300 proteins, including one never-before-described antimicrobial protein, dermcidin.16
Gustavo de Souza, M.D., identified an astonishing 491 proteins, both intra- and extra-cellular, using SDS-PAGE fractionation, in-gel trypsin digestion and independent analysis with two different high-performance mass spectrometers from a single healthy donor. Their study highlights the importance that proteases and protease inhibitors—crucial in wound healing and immune activity—may play in the eye’s maintenance of a healthy condition. Disturbance of the balance of these tear fluid proteins may lead to the development of different disease states.17 Thus, tear protein patterns may provide a way to distinguish dry eye sufferers from normal patients.
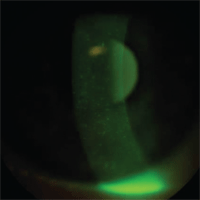
Dry eye.
Franz Grus, M.D., concluded that electrophoretic protein patterns using a surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) were more effective at detecting dry eye than standard clinical tests, such as Schirmer and tear film break-up time, with a specificity and sensitivity of 90%.18 The identification of these biomarkers revealed an increase in inflammatory markers and a decrease in some protective proteins.
Assumpta Peral, M.D., and colleagues found increased levels of adenosine molecules—AP4A and AP5A—in tears of dry eye patients as compared to controls.19 This finding corroborated the work by Jesus Pintor, M.D., and Gonzalo Carracedo, M.D., who found elevated diadenosine polyphosphates in dry eye and Sjögren’s patients.20,21
Ilias Alevizos, M.D., and colleagues were able to identify two microRNA patterns that were associated with salivary gland dysfunction and inflammation in Sjögren’s patients, representing another potential biomarker of that condition.22 Salivary gland biomarkers for Sjögren’s were also identified by Shen Hu, M.D. Three protein biomarkers (cathepsin D [CPD], -enolase and ß2-microglobulin [ß2m]) and three mRNA biomarkers (myeloid cell nuclear differentiation antigen [MNDA], guanylate binding protein 2 [GBP-2] and low-affinity IIIb receptor for the Fc fragment of IgG) were significantly elevated in patients with primary Sjögren’s when compared with both systemic lupus erythematosis patients and healthy controls.23
Mucin 1 (MUC1) protein is a membrane-spanning mucin of the ocular surface that is expressed by both corneal and conjunctival epithelia, and has been studied as a biomarker for breast cancer.24,25 Mucins provide lubricating and protective functions for the corneal epithelium and ocular surface.26 Barbara Caffery, O.D., Ph.D., and colleagues studied MUC1 protein expression in 76 patients: 25 with Sjögren’s Syndome (SS), 25 with keratoconjunctivitis sicca (KCS) and 26 non-dry eye controls. They found that patients in the SS group had significantly higher concentrations of soluble MUC1in their tears and MUC1 mRNA in their conjunctival epithelial cells as compared to the other two groups. It was also higher in the KCS group as compared to the controls. The authors hypothesize that this MUC1 expression could represent protective responses to chronic insult to the ocular surface.27 Dr. Caffery notes this may explain the increased amount of mucous in the tear film of SS patients that is often seen clinically.
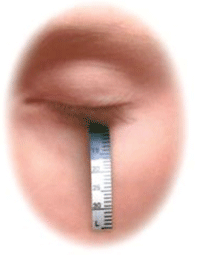
Schirmer strip testing.
Specific tear proteins have also been identified in meibomian gland disease (MGD). Louis Tong, M.D., evaluated 24 patients with dry eye disease as compared to 18 controls. Dry eye was defined as having dry eye symptoms with at least one abnormal finding on either Schirmer’s testing (without anesthetic), tear film break-up time or corneal staining. MGD was quantified on a 3 grade scale: 1 = lid margin telangiectasia, 2 = meibomian gland orifice plugging and 3 = both. Tear fluids were collected using Schirmer’s strips. Increased levels of S100A8 and S100A9 proteins were correlated with MGD severity in dry eye patients. They also found correlation between these protein levels and the patient reported symptoms of redness and transient visual blur.28
Early Diagnosis: Ocular Allergy
The incidence of allergic disease is rising to widespread proportions, which means that more people are increasingly looking to their eye care practitioners for rapid relief of red, watery, itchy eyes. While the more common seasonal and perennial ocular allergies are not sight-threatening conditions, they nonetheless have a major impact on an individual’s quality of life.29
Kaijin Ji, M.D., and colleagues studied the tear protein profiles of 21 seasonal and perennial allergic conjunctivitis patients and 13 controls. All patients completed a symptom questionnaire and underwent slit lamp examinations, with tear film break-up time, corneal staining and Schirmer testing, from which the tear sample was obtained. Analysis was performed via SDS-PAGE densitometry with soybean trypsin inhibitor (SBTI). The major protein tear bands were determined by mass spectrometry. They found that total tear protein concentrations and some of the major tear components (serum albumin precursor, Ig gamma-2 and leukocyte elastase inhibitor) were increased in the allergic conjunctivitis patients when compared to controls, as was a lipid-related protein secretory type IIa phospholipase A2 (sPLA2-IIa).30
Dongmei Chen, M.D., and colleagues at Mt. Sinai in New York have also reported on the increased sPLA2-IIa expression in allergic conjunctivitis patients—demonstrating that it is an inflammatory mediator when the ocular surface is compromised, as well as an antimicrobial agent.31
Early Treatment: Keratoconus
Catherine Pannebaker, O.D., and colleagues at Ohio State University enrolled 44 patients in a study to compare tear samples between patients with keratoconus and normal subjects.32 Tear samples were collected with a 5µl glass capillary tube from the inferior tear meniscus of all patients: 18 gas-permeable wearing keratoconus patients, six non gas-permeable wearing keratoconus patients and 20 gas-permeable wearing normals. Tears were evaluated via protein assay, cytokine antibody array, SDS-PAGE gels and mass spectrometry. They found increased expression of matrix-metalloproteinase-1(MMP-1) and tissue inhibitor of metalloproteinase 1 (TIMP-1) in keratoconic tears, and a decrease in tumor necrosis-related aptoptosis inducing ligand–R1 (TRAIL-R1) in the tears of gas-permeable wearing keratoconus patients when compared to normals.
Cyclosketal keratins, normally found in outer epidermis but not tears, found in both keratoconic groups. This finding suggested that keratoconus is associated with pathologic keratinization.32 MMP-1 is an enzyme that breaks down corneal collagens type I and III and has previously been noted in increased concentrations in keratoconic corneas, while increased levels of TIMP-1 and TIMP-2 may lead to the corneal scarring found in later stages of keratoconus, while being underexpressed in the clear corneas of early keratoconus.33-35 However, MMP-1 activity may be only intermittently expressed throughout the course of the disease, leading to variation in findings.36
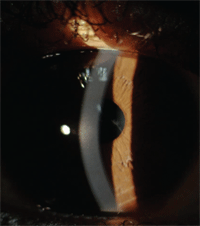
Bulging and scarring of keratoconus.
Isabel Lema, M.D., Ph.D., and colleagues used 2-D electrophoresis and mass spectrometry to identify proteins expressed in the tear film of keratoconic patients. They studied 22 patients with bilateral keratoconus, compared to 22 control subjects. Tear samples were collected by Schirmer testing. They isolated three proteins that were underexpressed in the tears of the keratoconic eyes: zinc-alpha2-glycoprotein (ZAG), lactoferrin and immunoglobulin kappa chain (IGKC).37 Tear lactoferrin is produced in the acinar cells of the lacrimal gland and is both antimicrobial and anti-inflammatory. It has been reported in the literature to be deficient in various ocular diseases, including Sjögrens Syndrome, vernal conjunctivitis and dry eye.38 ZAG’s exact role in tears is not well understood; the immunoglobulin kappa light chain is believed to play a role in the pathology of infectious and autoimmune diseases.39
The Impact of Biomarkers
While many biomarkers are proposed to be important determinants of disease risk, prognosis or even response to treatment, few of them eventually translate into improved clinical practice techniques.
Munson's sign.
John Ioannidis, M.D., and Orestis Panagioutou, M.D., evaluated 35 highly cited studies––30 of which reported a stronger association than observed in the largest single study of the same biomarker. When the largest studies of these biomarkers were examined, just 15 of the 35 original relationships were still clinically significant, and only half of those were clinically meaningful.40
George Poste, M.D., argued that while there are roughly 150,000 papers documenting thousands of claimed biomarkers, just 100 are routinely used in clinical practice. He postulated that a major impediment is a lack of standardization on how specimens are collected, handled and stored, as well as the insufficient numbers of samples per study—usually less than 100.41
In short, research to discover pertinent and clinically relevant biomarkers for commonly encountered ocular conditions is heating up and looks extremely promising. The ability and means to measure tear osmolarity in-office quickly and easily has already come to fruition. However, we need to be mindful that there is still much work to be done before we see research transition into an applicable, day-to-day clinical setting. It will truly be revolutionary when we can diagnose dry eye, ocular allergy and keratoconus via simple in-office objective tests that are independent of subjective symptoms, thus allowing the clinician to design individualized and targeted treatments.
Dr. Chaglasian is an associate professor at the Illinois College of Optometry in Chicago and an associate at Mack Eye Center in Hoffman Estates, Ill. The author would like to acknowledge DC Zagon for her assistance in preparing this article.
1. Nichols KK, Nichols JJ, Mitchell G. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004 Nov;23(8):762-70.
2. TFOS. 2007 Report of the International Dry Eye Workshop (DEWS). Ocul Surf. 2007;5(2):65-204.
3. Bron AJ, Yokoi N, Gaffney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009 Apr;7(2):78-92.
4. Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005 Sep;31(5):186-93.
5. Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007 May;26(4):452-60.
6. Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009 Aug;50(8):3671-9.
7. Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4309-15.
8. Farris RL. Tear osmolarity: a new gold standard? Adv Exp Med Biol. 1994;350:495-503.
9. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011 May;151(5):792-8.
10. Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6125-30.
11. Suzuki M, Massingale M, Ye F, et al. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vision Sci. 2010 Sep;51(9):4557-61.
12. Nelson JD, Farris RL. Sodium hyaluronate and polyvinyl alcohol artificial tear preparations. A comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 1988 Apr;106(4):484-7.
13. Tomlinson A, Khanal S, Ramaesh K, et al. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4309-15.
14. Khanal S, Tomlinson A, McFadyen A, et al. Dry eye diagnosis. Invest Ophthalmol Vis Sci. 2008 Apr;49(4):1407-14.
15. Green-Church KB, Nichols KK, Kleinholz NM, et al. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008 Mar 7;14:456-70.
16. You J, Fitzgerald A, Cozzi P, et al. Post-translation modification of proteins in tears. Electrophoresis. 2010 Jun;31(11):1853-61.
17. De Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7(8):R72.
18. Grus FH, Augustin AJ, Evangelou NG, Toth-Sagi K. Analysis of tear protein patterns as a diagnostic tool for the detection of dry eye. Eur J Ophthalmol. 1998 Apr-Jun;8(2):90-7.
19. Peral A, Carracedo G, Acosta MC, et al. Increased levels of diadenosine polyphosphates in dry eye. Invest Ophthalmol Vis Sci. 2006;47:4053-8.
20. Pintor J, Carracedo G, Alonso MC, et al. Presence of diadenosine polyphosphates in human tears. Pflugers Arch. 2002 Jan;443(3):432-6.
21. Carracedo G, Peral A, Pintor J. Diadenosine polyphosphates in tears of Sjögren syndrome patients. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5452-9.
22. Alevizos I, Alexander S, Turner RJ, Illei GG. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren’s syndrome. Arthritis Rheum. 2011 Feb;63(2):535-44.
23. Hu S, Gao K, Pollard R, et al. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res (Hoboken). 2010 Nov;62(11):1633-8.
24. Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995 Aug;36(9):1818-27.
25. Burchell J, Gendler S, Taylor-Papadimitriou J, et al. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987 Oct 15;47(20):5476-82.
26. Gipson IK, Inatomi T. Cellular origin of mucins of the ocular surface tear film. Adv Exp Med Biol. 1998;438:221-7.
27. Caffery B, Heynen ML, Joyce E, et al. MUC1 expression in Sjögren’s syndrome, KCS, and control subjects. Mol Vis. 2010 Aug 24;16:1720-7.
28. Tong L, Zhou L, Beuerman RW, et al. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol. 2011 Jun;95(6):848-52.
29. Alexander M, Berger W, Buchholz P, et al. The reliability, validity, and preliminary responsiveness of the eye allergy patient impact questionnaire (EAPIQ). Health Qual Life Outcomes. 2005;3:67.
30. Li K, Liu X, Chen Z, et al. Quantification of tear proteins and sPLA2-IIa alteration in patients with allergic conjunctivitis. Mol Vis. 2010 Oct 14;16:2084-91.
31. Chen D, Wei Y, Epstein S, et al. sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res. 2009 May;88(5):880-8.
32. Pannebaker C, Chandler HL, Nichols JJ. Tear proteomics in keratoconus. Mol Vis. 2010 Oct 2;16:1949-57.
33. Li DQ, Pflugfelder SC. Matrix metalloproteinases in corneal inflammation. Ocul Surf. 2005 Oct;3(4 Suppl):S198-20.
34. Seppälä HP, Määttä M, Rautia M, et al. EMMPRIN and MMP-1 in keratoconus. Cornea. 2006 Apr;25(3):325-30.
35. Kenney MC, Chwa M, Atilano SR, et al. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005 Mar;46(3):823-32.
36. Alexander CM, Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974-82.
37. Lema I, Brea D, Rodríguez-González R, et al. Proteomic analysis of the tear film in patients with keratoconus. Mol Vis. 2010 Oct 13;16:2055-61.
38. Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009 Jan;91(1):35-43.
39. Dugoujon J, Cambon-Thomsen A. Immunoglobulin Allotypes (Gm and Km) and their interactions with HLA antigens in autoimmune diseases: a review. Autoimmuninty. 1995;22(4):245-60.
40. Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. JAMA. 2011 Jun 1;305(21):2200-10.
41. Poste G. Bring on the biomarkers. Nature. 2011 Jan 13;469(7329):156-7.


