Drug administration methods are defined by how they generate therapeutic effects. Enteral drugs are swallowed and absorbed through the digestive system, while parenteral drugs are administered intravenously—both of these methods create systemic drug exposure.
However, many drugs can be applied directly to the tissues where the therapeutic benefit is needed. This is referred to as a topical administration. The major advantage to topically administered drugs is that a lower dosage is adequate to achieve a therapeutic effect, compared to the higher dosage that would be necessary if administered systemically. Moreover, topical administration of a locally acting medication minimizes the potential for systemic side effects.
Therefore, in considering the means available to treat allergy, there is a rationale favoring the use of anti-allergy medications applied directly to the eyes—with the intent to provide therapy for allergic rhinitis, as well as allergic conjunctivitis.
The Nasal-Ocular Connection
The physical relationship between the eye and nose favors the hypothesis that allergic rhinitis can be treated with an ophthalmically administered drug. Remember, the nasolacrimal ducts empty ocular secretions into the nasal cavity. Under normal circumstances, these ducts are patent allowing the free flow of fluids from the ocular surface into the nose.
As demonstrated in a clinical study enrolling 73 individuals with allergies, the movement of tears through the nasolacrimal ducts moves in one direction—from the ocular surface to the nose.1,2 In this trial, fluorescein dye was instilled in subjects’ eyes; soon thereafter the dye was detected in subjects’ nasal secretions.
But the opposite was not true. When fluorescein was sprayed up the nostrils, it was not detected in patients’ eyes to any significant degree. This result is probably due to the valves within the nasolacrimal apparatus—the valve of Rosenmuller and the valve of Hasner—which inhibit the reflux of fluids backwards from the nasal cavity into the eye.
Given the functionality of the nasolacrimal duct and its valves, it follows that an anti-allergy drug instilled in the eye would reach the nose. So, in theory, we can leverage this unidirectional flow to create therapeutic effects in the nasal cavity; however, this functional relationship might also create undesirable circumstances. For example, in allergic eyes that are exposed to environmental allergens, the antigenic particles as well as allergic mediators released from activated conjunctival mast cells would be present in tears and capable of reaching the nasal cavity to wreak inflammatory havoc.3,4
The Prevalence of Allergies
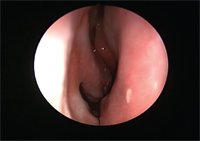
View of the nasal mucosa untreated and unsensitized.
Allergic diseases, and in particular, allergic rhinitis, are very common. It is estimated that allergic rhinitis affects 20 to 40 million people in the United States annually, including up to 30% of all adults.5
The signs and symptoms of allergic rhinitis are not limited to nasal tissues. In a clinical study enrolling 200 patients who received daily treatment for allergic rhinitis, 90.5% of study participants also reported experiencing ocular symptoms of allergy.6
Another study of 445 patients with symptoms of allergic conjunctivitis found that 95% of those had associated allergic diseases—primarily allergic rhinitis.7 Thus, nasal and ocular allergy symptoms are not mutually exclusive. In fact allergic rhinitis and allergic conjunctivitis could be considered one part of a broader allergic disease that includes a constellation of ocular and nasal symptoms.
Many patients visiting their eye care provider will have allergic sensitivities—seasonal allergies to grasses, weeds and tree pollen or perennial allergies to environmental allergens such as house dust mites, animal dander and chemicals. When allergens come in contact with the ocular tissues of allergic patients, a cascade of biochemical events are triggered through allergen-activation of mast cells, which creates allergic signs and symptoms in ocular and nasal tissues. Patients with seasonal allergies experience symptoms that correspond with pollen seasons. Some individuals have sensitivities to both perennial and seasonal allergens, and experience symptoms all times of the year.
Regardless of the type of allergy, the first means of disease management in patients should be environmental control measures—primarily, the avoidance of allergens and irritants to which patients are sensitive.5 Also fundamental to successful disease management is the use of medications that treat and prevent patients’ symptoms.5 For allergic rhinitis, a local or topical treatment is best. Treatment with a single drug, dosed once daily whenever possible, is desirable for simplicity and to maximize patient compliance. A topical agent can help minimize drug exposure and potentially reduce the occurrence of side effects that are associated with some systemically administered allergy medications.5,8
Preventative administration is a prudent strategy for topical allergy treatment. For optimal results, patients should take their medication prophylactically—before an anticipated allergen exposure—or on a regular basis as allergy maintenance therapy.5
Ophthalmic Meds: Steroids
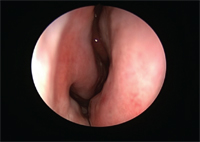
View of the nasal mucosa post Allergen Biocube (ABC) exposure.
Steroidal nasal sprays are commonly prescribed as maintenance therapy for many patients suffering from moderate to severe allergic rhinitis.5 In contrast, the use of ophthalmically applied steroids for allergy is limited. The ophthalmic medications Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb) and Pred Forte (prednisolone acetate 1%, Allergan) are effective for allergic conjunctivitis.
However, application of ophthalmic steroids has been associated with increased intraocular pressure after as little as two weeks of therapy.9 So, ocular steroids must be used with caution—particularly in patients with glaucoma—and administered for only short periods of time. A literature search failed to identify any studies examining the treatment effects of steroidal ophthalmic solutions in patients with allergic rhinitis.
Ophthalmic Meds: Antihistamines
Antihistamines are a popular choice in allergy medicines because they are considered very safe and histamine is a well-established target for allergy therapy. Upon its release from activated mast cells, histamine is the primary mediator for the most problematic signs and symptoms of allergy that occur in an allergic response. The binding of histamine to H1 histamine receptors located on the surface of neurons creates allergic itching.10 The binding of histamine to its cognate H1 and H2 receptors on the vascular endothelium initiates blood vessel dilation and congestion of adjacent tissues.11,12
In recent years, novel antihistamines have been developed for the treatment and prevention of ocular allergy symptoms. Of particular interest are the latest generation drugs having antihistaminic and mast cell-stabilizing properties. The newest antihistaminic ophthalmic solutions available by prescription with once-daily dosing include Pataday (olopatadine hydrochloride 0.2%, Alcon) and Lastacaft (alcaftadine 0.25%, Allergan).
The potency of these agents has provided the rationale to determine their effectiveness as treatments for the nasal signs and symptoms of allergy. Two environmental allergy studies with a total of 500 patients examined the effects of olopatadine hydrochloride 0.2% ophthalmic solution dosed once daily compared to placebo dosed once daily on patients’ nasal symptoms during the spring (10 week study) and fall (12 week study) allergy seasons.13
The results of the fall study demonstrated that, compared to placebo, treatment with olopatadine 0.2% eye drops significantly reduced the frequency of patients’ sneezing and itchy nose, and reduced the severity of patients’ sneezing, itchy nose and runny nose.
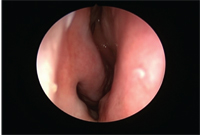
View of the nasal mucosa post-ABC exposure with antihistamine eye drop treatment.
The spring allergy study established that, compared to placebo treatment, olopatadine 0.2% treatment significantly reduced the frequency of pollen effects on sneezing and runny nose. In this study, olopatadine significantly reduced the frequency and/or severity of subjects’ nasal symptoms.13
These results are notable because this environmental clinical trial employed a similar design used with other drugs indicated for allergic rhinitis.14 That said, this trial and other environmental clinical studies have many uncontrolled variables including differing allergenic exposures present at each subject’s home and workplace, as well as varying degrees of allergen exposure during peak pollen days.
A more well-controlled clinical model for allergy is the conjunctival allergen challenge (CAC), which has been used to evaluate the efficacy of all U.S. Food and Drug Administration-approved prescribed medications for allergic conjunctivitis currently on the market.15 During a CAC, allergen is applied to the conjunctiva of allergic patients creating ocular itching, conjunctival redness, excessive tearing and chemosis, as well as nasal, ear and palate itching, rhinorrhea, sneezing and nasal congestion. CAC studies are typically designed to record subjects’ ocular and nasal allergic symptoms scored by the subject, and ocular and nasal allergic signs scored by study investigators.
One such double-blind CAC study enrolled allergic subjects who were treated with olopatadine hydrochloride 0.1% ophthalmic solution b.i.d., 50µg q.d. mometasone furoate monohydrate nasal spray or 180mg q.d. fexofenadine hydrochloride tablets for one week.2 The study results demonstrated these three treatment regimens provided similar levels of prevention against sneezing, rhinorrhea/post-nasal drip, nasal itching, palate itching and nasal congestion elicited by conjunctival allergen exposure.2 Since this study was conducted, olopatadine has been re-formulated to a new ophthalmic solution in Pataday (olopatadine hydrochloride 0.2%, Alcon) that is more concentrated than the original eye drop, Patanol (olopatadine hydrochloride 0.1%, Alcon) and can be dosed once daily. It is also available as a prescription nasal spray, Patanase (olopatadine 0.6%, Alcon) that is dosed twice daily; these olopatadine-containing drugs are indicated to treat itching associated with allergic conjunctivitis and allergic rhinitis, respectively.
In July 2010, the FDA approved Lastacaft (alcaftadine 0.25%, Allergan) for the prevention of itching associated with allergic conjunctivitis. Like olopatadine, alcaftadine is a dual-acting H1 antihistamine. Studies have shown that allergen-induced degradation of zonula occludin 1 and E-cadherin within the conjunctival epithelium of test animals was reduced by treatment with alcaftadine, suggesting that alcaftadine works by protecting epithelial tight junctions and reducing allergen load, and subsequently minimizing the release of preformed allergic mediators from mast cells and blocking the activation of histamine receptors in ocular tissues.16
The CAC model has also been used to characterize the anti-allergy properties of alcaftadine. A proof-of-concept CAC clinical study compared the anti-allergy activities of alcaftadine 0.25% treatment and olopatadine hydrochloride 0.1% treatment with placebo.17 The study included as secondary endpoints patients’ nasal symptom composite scores and individual symptom scores for sneezing, rhinorrhea, nasal congestion, nasal itching, and ear or palate itching. When measured 15 minutes and 16 hours after drug instillation, both the alcaftadine and olopatadine treatment groups experienced significantly improved nasal symptoms composite scores at each time point compared to the placebo group.
Gail Torkildsen, M.D., and Arthur Shedden, M.D., have also reported the treatment effects of alcaftadine 0.25% ophthalmic solution on patients’ nasal symptoms following conjunctival allergen challenge.18 In a multi-center study enrolling 60 patients, one of the secondary study endpoints was nasal signs and symptoms reported as a composite of individual scores for rhinorrhea, nasal congestion, nasal itching, ear and/or palate itching. When dosed 15 minutes or 16 hours prior to allergen challenge, patients treated with alcaftadine demonstrated statistically significant improvements in nasal composite scores compared to patients receiving a placebo eye drop. These improvements over placebo were greater 16 hours after dosing than 15 minutes after dosing, demonstrating a duration of action for alcaftadine of at least 16 hours.18
A Combination of the Two?
Some patients will not experience complete relief of their nasal allergy symptoms with the use of an ophthalmic anti-allergy medicine, and will likely benefit from a combination drug regimen. Using the CAC clinical model, a number of studies have demonstrated treatment benefits when an ophthalmic medication was added to either a steroidal nasal spray or an orally administered antihistamine.6,19-20
It is advantageous to treat allergic disease locally with a topically-applied ophthalmic medication. Ophthalmic allergy treatment can provide patients with rapid relief of ocular allergy signs and symptoms, as well as treat nasal symptoms. For patients with mild to moderate allergic rhinitis, it may be best to think about a preventative treatment strategy and start with a topical ophthalmic antihistamine. For patients already taking prescribed pills or nasal sprays for allergy but still experiencing persistent ocular or nasal symptoms, secondary treatment with an ophthalmic solution can help.
Dr. Abelson is a Clinical Professor of Ophthalmology at Harvard Medical School and Senior Clinical Scientist at Schepens Eye Research Institute.
1. Sisler HA. One-way flow in the lacrimal drainage system: determinants from the basic sciences. Ann Ophthalmol 1982 Jan;14(1):76-7.
2. Spangler DL, Abelson MB, Ober A, Gomes PJ. Randomized, double-masked comparison of olopatadine ophthalmic solution, mometasone furoate monohydrate nasal spray, and fexofenadine hydrochloride tablets using the conjunctival and nasal allergen challenge models. Clin Ther. 2003 Aug;25(8):2245-67
3. Abelson MB, Soter NA, Simon MA, et al. Histamine in human tears. Am J Ophthalmol. 1977 Mar;83(3):417-8.
4. Abelson MB, Allansmith MR. Histamine in the eye. In: Silverstein A, O’Connor G, eds. Immunology and Immunopathology of the Eye. New York: Masson Publishing, 1979.
5. Dykewicz MS, Fineman S, Nicklas R, et al. Joint Task Force Algorithm and Annotations for Diagnosis and Management of Rhinitis. Ann Allergy Asthma Immunol. 1998 Nov;81(5 Pt 2):469-73.
6. Berger W, Abelson MB, Gomes PJ, et al. Effects of adjuvant therapy with 0.1% olopatadine hydrochloride ophthalmic solution on quality of life in patients with allergic rhinitis using systemic or nasal therapy. Ann Allergy Asthma Immunol. 2005 Oct;95(4):361-71.
7. Kosrirukvongs P, Visitsunthorn N, Vichyanond P, Bunnag C. Allergic conjunctivitis. Asian Pac J Allergy Immunol. 2001 Dec;19(4):237-44.
8. Casale TB, Blaiss MS, Gelfand E, et al. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003 May;111(5):S835-42.
9. Berdy GJ, Stoppel JO, Epstein AB. Comparison of the clinical efficacy and tolerability of olopatadine hydrochloride 0.1% ophthalmic solution and loteprednol etabonate 0.2% ophthalmic suspension in the conjunctival allergen challenge model. Clin Ther. 2002 Sep;24(6):918-29.
10. Taylor-Clark TE, Kollarik M, MacGlashan DW, Jr., Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol. 2005 Dec;116(6):1282-8.
11. Abelson MB, Udell IJ. H2-receptors in the human ocular surface. Arch Ophthalmol. 1981 Feb;99(2):302-4.
12. Nakano Y, Takahashi Y, Ono R, et al. Role of histamine H(4) receptor in allergic conjunctivitis in mice. Eur J Pharmacol. 2009 Apr;608(1-3):71-5.
13. Abelson MB, Gomes PJ, Vogelson CT, et al. Effects of a new formulation of olopatadine ophthalmic solution on nasal symptoms relative to placebo in two studies involving subjects with allergic conjunctivitis or rhinoconjunctivitis. Curr Med Res Opin. 2005 May;21(5):683-91.
14. Nayak AS, Philip G, Lu S, et al. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002 Jun;88(6):592-600.
15. Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990 Jan;108(1):84-8.
16. Ono SJ, Lane K. Comparison of effects of alcaftadine and olopatadine on conjunctival epithelium and eosinophil recruitment in a murine model of allergic conjunctivitis. Drug Design, Development and Therapy. 2011;5:1-8.
17. Greiner J, Edwards-Swanson K, Ingerman A. Evaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1%. Clinical Ophthalmology. 2011;5:87-93.
18. Torkildsen G, Shedden A. The safety and efficacy of alcaftadine 0.25% ophthalmic solution for the prevention of itching associated with allergic conjunctivitis. Curr Med Res Opin. 2011;27(3):623-31.
19. Lanier BQ, Abelson MB, Berger WE, et al. Comparison of the efficacy of combined fluticasone propionate and olopatadine versus combined fluticasone propionate and fexofenadine for the treatment of allergic rhinoconjunctivitis induced by conjunctival allergen challenge. Clin Ther. 2002 Jul;24(7):1161-74.
20. Crampton HJ. Comparison of ketotifen fumarate ophthalmic solution alone, desloratadine alone, and their combination for inhibition of the signs and symptoms of seasonal allergic rhinoconjunctivitis in the conjunctival allergen challenge model: a double-masked, placebo- and active-controlled trial. Clin Ther. 2003 Jul;25(7):1975-87.


