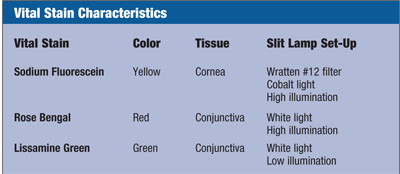
Vital stains are some of the best tools practitioners can use to assess the health and integrity of the ocular surface. They are relatively non-invasive, efficient and economical. This article will review the three stains commonly used in clinical practice: sodium fluorescein, lissamine green and rose bengal. I will give some general rules of thumb for optimizing their use, point out the advantages and disadvantages for each one and convince you that every practice should routinely use them.
A Historical Perspective
Staining is a term that describes the epithelial disruption and other pathophysiologic changes observed when using topical dyes––also referred to as vital stains.1 Vital stains allow visualization of tissues in their living state.2 Pflüger first described sodium fluorescein, often referred to simply as fluorescein, which he used to stain the cornea and conjunctiva in rabbits in 1882.3 Until Henrik Sjögren, M.D., introduced rose bengal in 1933, fluorescein was also the primary stain used to stain the conjunctiva.4 In 1973, Mogens Norn, M.D., introduced lissamine green as a vital stain with staining properties almost identical to those of rose bengal.5
Clinical Use and Methodology
Corneal epithelial staining is best observed with fluorescein, while conjunctival staining is best observed with lissamine green or rose bengal. In clinical practice, the use of individually wrapped, sterile, dye-impregnated paper strips is the preferred staining technique. The method is straightforward: apply a small drop of sterile saline to the tip of the paper strip, instruct the patient to look up and lightly apply the strip to the inferior palpebral or bulbar conjunctiva. The inferior palpebral conjunctiva is preferred due to Bell’s phenomenon, the normal reflex of upward and outward eye movements observed during a blink or when approaching the eye.6 In some cases, it may be possible to drop the saline/dye solution from the strip without touching the ocular surface. For patients with reduced tear volume associated with severe dry eye, it may be necessary to carefully instill the dye on the superior bulbar conjunctiva so that gravity may help distribute the stain over the ocular surface. To avoid a corneal abrasion, never apply a paper strip to the cornea.
For research purposes, vital stains are typically obtained from pharmacies in preservative-free bottles, and then instilled in liquid form using a pipette. By using a pipette for instillation, the inherent variability of dye concentration and volume applied to the ocular surface with use of paper strips is minimized.7 However, preservative-free bottles of vital stains are not recommended in clinical practice due to the potential risk of Pseudomonas aeruginosa growth.8
Be consistent with evaluating staining the same amount of time after dye instillation because staining can vary considerably depending on the time between instillation and observation. Fluorescein and lissamine green staining are best observed two minutes after instillation.9,10 The time after dye instillation to assess staining is critical because an assessment made too soon or too late will result in a staining score that is lower than the maximum staining potential.10 If solution toxicity or solution/lens interactions are suspected, staining is best observed one to two hours after insertion of contact lenses.11
After the slit lamp exam, always sketch the pattern of any staining observed, note how much staining was present and the type of dye that was used. A sketch is helpful for comparing the staining observed at subsequent clinic visits.
In your notes, staining should be characterized by: pattern (focal, diffuse, punctate or coalesced); position (superior, inferior, nasal, temporal or central); depth (superficial or deep); and grade (e.g. 0 = none, 1= trace, 2 = mild, 3 = moderate, 4 = severe). Whether you choose a 0-3, 0-4 or another scale for your measurements, be sure to use a consistent metric in your practice. When writing referral or consultation letters, use notation that includes not only the grade of staining, but also a description of what the grade represents (e.g., grade 2-mild).
Sodium Fluorescein
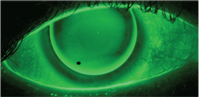
Evaluation of an RGP contact lens fit with sodium fluorescein.
Sodium fluorescein permeates into the intercellular space associated with any epithelial cellular disruption.12,13 Fluorescence should be observed with a blue excitation filter over the white light source and can be highlighted by placing a Wratten #12 yellow filter over the slit-lamp objective. Fluorescein is highly tolerable and a single application does not cause stinging. However, a disadvantage to using fluorescein is that the fluorescent tear film may obscure corneal staining measurements.14 To avoid inaccurate staining measurements, conduct tear film assessments (tear film break up time and tear film meniscus height) first, allowing time for the fluorescein to diffuse from the tear film to the cornea, then measure corneal staining.15
It is not uncommon to observe minimal amounts of corneal staining in normal, healthy eyes; however, staining associated with contact lens wear should be carefully evaluated.16 Nathan Efron, D.Sc., classified staining associated with contact lens wear into six etiologic categories: mechanical, exposure, metabolic, toxic, allergic and infections.1 While a myriad corneal staining patterns may occur, the staining pattern itself can be indicative of the underlying cause. However, when I am sitting behind the slit lamp, I can hear my disease professor at the University of Missouri–St. Louis School of Optometry say, “When you hear hoof beats, think horses... not zebras.” In other words, the obvious diagnosis is correct most of the time.
 Some examples of the more common corneal staining patterns include:
Some examples of the more common corneal staining patterns include:
• Superficial punctate keratitis (SPK). SPK can present as isolated or diffuse dots across a larger area of the cornea. An isolated pattern may be due to a poor contact lens fit or infection, while a diffuse pattern may indicate solution toxicity or an interaction between certain lens/solution combinations or dry eye syndrome.
• Superior epithelial arcuate lesion (SEAL). SEALs present parallel to the superior limbus and are due to mechanical chaffing by a contact lens on the superior cornea. The patient is usually asymptomatic.
• Inferior arcuate staining. Inferior arcuate staining presents parallel to the inferior limbus and is due to dehydrated contact lenses associated with insufficient post-lens tear film. Patients may have complaints of mild discomfort.
• Three and nine o’clock staining. Three and nine o’clock staining presents parallel to the nasal and temporal limbus and is due to contact lens associated dry eye. Patients may experience mild discomfort.
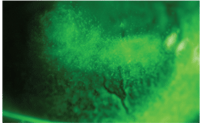
• Dimple veil staining. Dimple veil staining is not true staining, but rather pooling of the dye into corneal indentions caused by trapped air bubbles under a poorly fitting rigid gas permeable contact lens. It presents as sharply demarcated, circular patterns of stain on the cornea.
• Foreign body staining. Staining from a foreign body or mechanical trauma can present in various ways, such as a zigzag-shaped abrasion or as the outline shape of an embedded foreign body.
• Epithelial basement membrane dystrophy (EBMD). EBMD is prevalent in approximately 2% to 6% of patients. It presents as raised dots, maps and fingerprints and results in negative staining. Both EBMD and dry eye syndrome may cause reduced tear film break-up time; however, the elevations of the ocular surface associated with EBMD result in an immediate tear film break-up over the corresponding area, while dry eye syndrome results in a delayed tear film break-up. Patients are usually asymptomatic unless the dots, maps and fingerprints erupt, at which time they will stain positively and become symptomatic.17,18
• Herpetic keratitis. Dendritic ulcers are associated with the herpes simplex virus (HSV). The edges may be slightly elevated due to swollen epithelial cells.
Not only useful for staining, fluorescein is also used to fluoresce the tear film when measuring intraocular pressure with applanation tonometry, measuring tear film break-up time, assessing the fit of a rigid gas permeable contact lens and detecting aqueous humor leakage from the anterior chamber. It is also beneficial for highlighting papillary conjunctivitis by settling in between the individual papillae. In addition, by instilling a small amount of fluorescein in the tear film, the tear volume is more easily observed when evaluating the height of the tear film meniscus.19
Lissamine Green
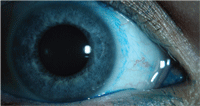
Contact lens induced conjunctival
staining with lissamine green.
Lissamine green is an acidic, synthetically produced, organic dye that has been historically used in food products.5,20 Since the introduction of lissamine green, clinical reports have indicated its use as a stain to diagnose ocular surface disease. Lissamine green stains dead and degenerate cells, yet does not stain healthy epithelial cells.21 The literature contains no reports of toxicity, and, at 1% concentration, this dye is not associated with stinging or discomfort. Studies have shown that lissamine green and rose bengal have similar staining profiles, yet lissamine green is better tolerated by patients.9,22
Lissamine green should be your preferred dye for staining the bulbar conjunctiva. Additionally, lissamine green staining can be used to detect early dry eye. A study conducted at The University of Texas Southwestern Medical Center found that three staining patterns can indicate the progression of dry eye severity levels: first, the nasal conjunctiva stains; next, the nasal and temporal conjunctiva stains; and finally, the cornea, nasal and temporal conjunctiva stains.23
Conjunctival staining is useful for evaluating both dry eye patients and contact lens wearers. In contact lens patients, circumlimbal staining observed with lissamine green may be an indicator of contact lens induced conjunctival staining (CLICS)—indicating that the lens is too tight or has a sharp edge design. The patient may need to be refit into a different lens with a flatter base curve or a different edge design.24 When a lens is too tight, the mechanical force of the lens’ edge may cause an indention in the conjunctiva, which may be observed by pooling of fluorescein or lissamine green in the indentation. A patient having CLICS is usually asymptomatic and the ocular surface may otherwise be clear; however, the consequences of continuous mechanical friction in the circumlimbal area are unknown.
In patients who present with dry eye symptoms in the absence of dry eye findings, lissamine green can be used to evaluate the superior and inferior lid margins for lid wiper epitheliopathy.9 It is also a useful dye for evaluating herpetic lesions associated with the herpes simplex virus and neoplastic lesions, as well as for diagnosing keratoconjunctivits sicca (KCS).25 A differential diagnosis for KCS is of particular importance because patients with Sjögren’s syndrome, tested objectively by the presence of xerostomia and KCS, have a nine times higher prevalence of autoimmune thyroid disease.26
Rose Bengal

Dimple veil staining with sodium
fluorescein.
Rose bengal stains dead and devitalized cells, as well as mucus, and should be observed using a white light source.12 Mucins and other tear film components block rose bengal staining, thus a break in the tear film must be present for rose bengal to penetrate the ocular surface.27 Prior to the introduction of lissamine green in 1973, rose bengal was the preferred dye for assessing conjunctival staining. However, it is widely known that rose bengal causes stinging—sometimes severe—in most patients and is mildly toxic to the ocular surface.22,28-30 In addition, rose bengal stains healthy epithelial cells and, therefore, is not technically considered a “vital” dye.21,28 Based on its shortcomings, rose bengal has been losing its popularity in clinical practice.
Words of Wisdom
Sjögren’s Syndrome with sodium
fluorescein.
The use of vital stains—particularly fluorescein and lissamine green—should be a common tool in your practice. Use fluorescein to stain the cornea and lissamine green to stain the conjunctiva even in your asymptomatic patients. There may be some situations that warrant the use of rose bengal, but carefully consider if lissamine green may be a better choice. Be familiar with the various patterns of staining that may occur with common ocular surface disruptions, use the pattern to determine the etiology, and then manage it appropriately.
A last bit of advice: It may be helpful for you to know that fluorescein, rose bengal and lissamine green are all water soluble dyes. I remember accidentally spilling fluorescein on a patient’s shirt my first year practicing. The patient was very upset, and I was mortified. Fortunately, the patient called the next day to let me know the fluorescein washed out of her shirt. I have since conducted my own at-home experiment to evaluate the application of each of these three dyes on a white 100% cotton fabric t-shirt…and they all washed out.
Dr. Ramsey is an Assistant Director of Clinical Trial Management for Alcon Research, Ltd. She is a fellow of the American Academy of Optometry and a member of the American Optometric Association. Prior to joining Alcon, she practiced optometry in Salt Lake City, Utah. She is a 2001 graduate of the UM-St. Louis School of Optometry and a 2009 graduate of the Masters of Clinical Vision Research program at Nova Southeastern University.
1.Efron N. Contact Lens Complications. 2nd ed. London, United Kingdom: Butterworth-Heinemann; 2004.
2. Ehrlic P. Ueber die Methylenblaureaction der lebenden Nervensubstanz. Biol Centralbl. 1886-87;6;214-24.
3. Pflüger. Zur Ernahrung der Cornea. Klin Monatsbl Augenheilkd. 1882;20:69-81.
4. Sjögren H. Zur Kenntnis der Keratoconjunctivitis sicca (Keratitis filiformis bei Hypofunktion der Tranendrusen). Acta Ophthalmol Suppl. 1933 Mar:13(1-2):40-5.
5. Norn M. Lissamine green. Vital staining of the cornea and conjunctiva. Acta Ophthalmol (Copenh). 1973;51(4):483-91.
6. Jones DH. Bell’s phenomenon should not be regarded as a pathonomonic sign. BMJ. 2001 Oct;323(7318):935.
7. Snyder C, Paugh JR. Rose bengal dye concentration and volume delivered via dye-impregnated paper strips. Optom Vis Sci. 1998 May;75(5):339-41.
8. Vaughan DG. The contamination of fluorescein solutions. Am J Ophthalmol. 1955 Jan;39(1):55-61.
9. Korb DR, Herman JP, Blackie CA, et al. Prevalence of lid wiper epitheliopathy in subjects with dry eye signs and symptoms. Cornea. 2010 Apr;29(4):377-83.
10. Ramsey A, Seger K, Proskin H. Defining the Conjunctival Staining Method: Instillation Volume and Time Course to Assess Staining with Lissamine Green. Poster presented at: American Academy of Optometry. 2009: Orlando, FL.
11. Garofalo R, Dannasanayake N, Carey C, et al. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye Contact Lens. 2005 Jul;31(4):166-74.
12. Norn M. External Eye. Methods of Examination. Copenhagen, Denmark: Scriptor; 1974.
13. Romanchuk KG. Fluorescein. Physiochemical factors affecting its fluorescence. Surv Ophthalmol. 1982 Mar-Apr;26(5):260-83.
14. Bron A, Evans V, Smith J. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640-50.
15. Abelson M, Ousler G, Nally L, Emory T. Dry Eye Syndromes: Diagnosis, Clinical Trials and Pharmaceutical Treatment - ‘Improving Clinical Trials’. In: Lacrimal Gland, Tear Film, and Dry Eye Syndromes. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2002:1079-86.
16. Norn M. Micropunctate fluorescein vital staining of the cornea. Acta Ophthalmol. 1970;48: 108-18.
17. Catania L. Primary Care of the Anterior Segment. 2nd ed. Stamford, CT: Appleton & Lange; 1996.
18. Hom M, Bruce A. Manual of Contact Lens Prescribing and Fitting. 3rd ed. 2006.
19. Mainstone J, Bruce A, Golding T. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996 Jun;15(6):653-61.
20. Norn M. Diagnosis of Dry Eye. In: Lemp M, Marquardt R. The Dry Eye. A Comprehensive Guide. Berlin, Germany: Springer-Verlag; 1992:167.
21. Kim J, Foulks G. Evaluation of the effect of lissamine green and rose bengal on human corneal epithelial cells. Cornea. 1999 May;18(3):328-32.
22. Manning F, Wehrly S, Foulks G. Patient tolerance and ocular surface staining characteristics of lissamine green versus rose bengal. Ophthalmol. 1995 Dec;102(12):1953-7.
23. Uchiyama E, Aronowicz JD, Butovich IA, McCulley JP. Pattern of vital staining and its correlation with aqueous tear deficiency and meibomian gland dropout. Eye Contact Lens. 2007 Jul;33(4):177-9.
24. Garofalo R, Ramsey A. Going green to evaluate contact lens fit. CL Spectrum. 2010;25(5):34-7.
25. Norn M. Vital staining of the cornea and conjunctiva. Acta Ophthalmol.1962;40:389-401.
26. Hansen BU, Ericsson UB, Henricsson V, et al. Autoimmune thyroiditis and primary Sjogren’s syndrome: clinical and laboratory evidence of the coexistence of the two diseases. Clin Exp Rheumatol. 1991 Mar-Apr;9(2):137-41.
27. Khan-Lim D, Berry M. Still confused about rose bengal? Curr Eye Res. 2004 Oct-Nov;29(4-5):311-7.
28. Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmol. 1992 Apr;99(4):605-17.
29. Feenstra RP, Tseng SC. What is actually stained by rose bengal? Arch Ophthalmol. 1992 Jul;110(7): 984-93.
30. Korb DR. Survey of preferred tests for diagnosis of the tear film and dry eye. Cornea. 2000 Jul;19(4):483-6.


