Ocular allergy is one of the most rapidly developing disease states seen in primary eye care. There have been several proposed etiologies for this increased rate, such as climate change, dietary patterns and the “hygiene hypothesis”—a theory that attributes the rise to a decrease in the normal environmental stimuli required to activate a healthy immune system secondary to a decrease in outdoor activities in childhood and the subsequent inappropriate stimulation of the IgE aspect of the immune system.1
Because of this rise in ocular allergies, all clinicians need to be comfortable with both diagnosing and treating the entire range of allergic disease. Seasonal allergies have increased over the last several decades; for ocular allergies, clinicians have found topical antihistamine/mast cell stabilizing agents to be most effective. We have also seen loteprednol begin to play a significant role in recent years.
There are several additional complex allergies with associated ocular complications, such as atopic keratoconjunctivitis, which can demonstrate periorbital dermatitis and significant inflammatory cascade, and requires steroid therapy or immunomodulating agents like tacrolimus or promiculimus.
A significant but less frequent variant of allergic ocular disease is vernal conjunctivitis (VKC). This article reviews its symptoms and treatments.
What is Vernal Conjunctivitis?
Vernal conjunctivitis, a condition most commonly seen in younger males between the ages of three and 20, is frequently associated with atopic disease. Seasonal activity is usually observed in the Northern hemisphere, but it can be a perennial disease in warmer climates. It is a persistent condition—half of patients show significant clinical activity at five years.2
Ocular complications involve early stage changes such as enlarged papillae of the upper lid that can develop into plaque-like structures that play a role in the pathophysiology of the corneal findings. This occurs as a result of mechanical debridement of the epithelium as the plaques enlarge. Additional early signs include bilateral inflammation of the upper limbal area, Horner-Trantas dots, limbal follicles and thick mucin-based discharge.
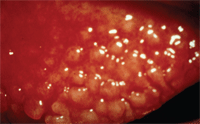
Developing tarsal papillae.
As the disease progresses, corneal involvement typically develops; it can present anywhere from mild superficial punctate keratitis (SPK) to advanced shield ulcers. One study showed corneal involvement in up to 50% of treated patients.3
The disease shows a predilection for increased limbal involvement in patients who live in warmer climates and those who have increased epidermal pigment. This usually involves conjunctival hyperemia and papillae at the limbal junction, along with Horner-Trantas dots.4 Some patients demonstrate a pannus that invades the cornea from the superior limbal arcade and can lead to severe vision loss if not treated adequately.
Shield ulcers are common in more advanced cases and are etiologically related to both mechanical irritation by the upper lid plaques as well as a complex inflammatory response that recruits eosinophils to the ocular surface.
Conjunctival changes include subconjunctival fibrosis, keratinization and symblepharon.2-4 Clinical complications of this type are uncommon in northern climates, but are seen in geographies closer to the equator.
What is the Treatment?
• Antihistamine/mast cell stabilizers. Vernal conjunctivitis is best treated in its earliest presentation with topical antihistamine/mast cell stabilizing agents. These should be used during the entire allergy season, or year-round if the condition is perennial. This therapy is effective as a baseline intervention, but is frequently insufficient as the disease worsens. At that point, a topical steroid is the best choice.
• Topical steroids. Steroid dosage varies based on the severity of symptoms, and the level and duration of the disease. In a comparison study looking at prednisolone, fluorometholone and loteprednol, researchers found no difference in clinical efficacy between the agents but did find a notable IOP spike in the prednisolone group.5
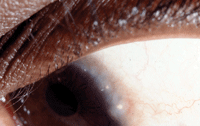
Horner-Trantas dots.
• Cyclosporine. Several authors are currently investigating the use of cyclosporine over prolonged periods of time. One study of 2,597 patients with VKC using cyclosporine therapy reported a significant decrease in symptoms at the six-month follow up. In addition, 30% of topical steroid users were able to discontinue use within three months of cyclosporine therapy.6
Another study monitored 156 children with VKC who were treated with either a 1% or 2% concentration of cyclosporine two to three times per day for up to seven years; patients reported a very good response and minimal side effects.7 Due to the increased concentration of the drug vs. what is available in standard commercial preparations, yearly lab tests were conducted to monitor kidney and liver functions as well as serum levels. No notable levels were recorded over the duration of the study.7
Stepwise by Severity
• Initial therapy. In my practice, I initiate treatment with antihistamine/mast cell agents BID, followed by Lotemax (loteprednol, Bausch + Lomb) if available on the patient’s formulary, or generic fluorometholone alcohol 0.1%. Dosage varies from QID to QH in advanced cases. I taper the steroid therapy over several weeks once the disease state is under control.
• Persistent cases. If the patient has a previous history of the disease or is slow to respond to the initial therapy, I institute cyclosporine treatment QID and taper the steroid over a longer period of time. This treatment protocol, reviewed by Osmo Kari, MD, and K. Matti Sarri, MD, was found very effective in managing recurrent or perennial patients.8
• Corneal involvement. If the disease still progresses and the patient develops corneal involvement, more aggressive intervention is required to prevent vision loss. In most cases, I increase the intensity of the steroid by switching to an agent like Durezol (difluprednate hydrochloride 0.05%, Alcon) while maintaining the cyclosporine and antihistamine agents. The goal is to prevent corneal complications at all costs, which is achieved by targeting the plaque-like papillae on the upper tarsal plate.
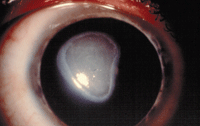
Shield ulcer.
In patients with SPK, institute bandage contact lens (BCL) therapy to give protection against the low-grade trauma from the upper lid.9 Silicone hydrogel BCLs are excellent choices for the average VKC patient; in addition, published cases recommend using larger diameter lenses (e.g., 22mm hydrogel, Contaflex T75) in individuals with both corneal and conjunctival complications.10
• Shield ulcers. If shield ulcers develop, aggressive therapy includes maximizing the anti-inflammatory therapy, increasing Durezol to Q2H and fitting an appropriate diameter BCL with prophylactic antibiotic to reduce the potential for secondary infection. Remember to debride mucus plaques before using BCLs or immediately after they appear.11 Also start homatropine 5% BID to TID (dependent on iris color) to minimize the impact of the photophobia commonly associated with this stage of the disease. Patients at this level need to be monitored carefully for disease progression and any signs of infection that might include an infiltrative response or increased pain, and complications in therapy, such as IOP spikes.
• Non-responsive cases. The use of topical 0.005% tacrolimus has been shown to be a reasonable alternative to steroid therapy in non-responsive patients.12 In rare instances the cornea will either not respond to the above treatments or develop additional complications, such as limbal stem cell disease (LSCD), as a result of the VKC. One study evaluated 2,225 VKC patients, of which 49 eyes were noted to have LSCD complications related to VKC. Interestingly, just half of the eyes developed this complication in the active phase of the disease. Treatment in the study included the use of amniotic membrane graft.13
In these non-responsive cases, clinicians have the option to use a new commercial product, ProKera (BioTissue). It is constructed of preserved human amniotic membrane (AmnioGraft, BioTissue) that is clipped into a dual PMMA scleral ring. ProKera has been reportedly effective for a variety of non-healing corneal lesions, including the indicated shield ulcers.14 Personally, I have found repeated success with the ProKera system and would recommend it as a viable alternative in patients at risk of vision loss who are non-responsive to more traditional therapy.
The management of VKC is best accomplished by vigilance in observation and treatment of the disease. In advanced cases, you may need to see the patient daily during the initial phase of therapy, with weekly monitoring for individuals who have responded well or have a previous history and are reliable to be monitored by phone after initial consultation.
Stay aggressive at every level of therapy to minimize cellular damage from repeated episodes that could progress to vision loss over time, but also reduce dosing when possible to avoid complications associated with prolonged steroid usage. Patients may benefit from desensitization therapy when the disease progresses in spite of maximum clinical intervention.
Finally, a collaborative team approach with the eye care practitioner, pediatrician and allergist is needed to effectively treat VKC.
Dr. Thimons graduated from Ohio State University College of Optometry and has served as chief, Optometric Service at the VA Medical Center in Chillicothe, center director and chairman at Omni Eye Services in Fairfax, Va., director at the Glaucoma Institute at SUNY and executive director at TLC Connecticut. In 2002, he founded the Ophthalmic Consultants of Connecticut and serves as ophthalmic medical director.
1. Wordwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998 Apr;351(9111):1225-32.
2. Bonini S, Bonini S, Shiavone M, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term follow-up. Ophthalmology. 2000 Jun;107(6):1157-63.
3. Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004 Apr;18(4):345-51.
4. Hall A, Shilio B. Allergic conjunctivitis. Community Eye Health J. 2005 Mar;118(53):76-8.
5. Oner V, Türkcü FM, Tas M, et al. Topical loteprednol etabonate 0.5% for traetment fo vernal keratoconjunctivitis: efficacy and safety. Jpn J Ophthalmol. 2012 Jul; 56(4):312-8.
6. Takamura E, Uchio E, Ebihara N, et al. A prospective, observational, all-prescribed-patients study of cyclosporine 0.1% ophthalmic solution in the treatment of vernal keratoconunctivitis. Nihon Ganka Gakkai Zasshi. 2011 Jun;115(6):508-15.
7. Pucci N, Caputo R, Mori F, et al. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010 Jul-Sep;23(3):865-71.
8. Kari O, Saari KM. Updates in the treatment of ocular allergies. J Asthma/Allergy. 2010 Nov;24(3):149-58.
9. Lemp M. Contact lenses and allergy. Curr Opin Allergy Clin Immunol. 2008;8(5):457-60.
10. Quah SA, Hemmerdinger C, Nicholson S, Kaye SB. Treatment of refractory vernal ulcers with large-diameter bandage contact lenses. Eye Contact Lens. 2006 Sep;32(5):245-7.
11. Caputo R, Pucci N, Mori F, et al. Surgical debridement plus topical cyclosporine A in the treatment of vernal shield ulcers. Int J Immunopathol Pharmacol. 2012 Jul-Sep;25(3):775-80.
12. Kheirkhah A, Zavareh MK, Farzbod F, et al. Topical 0.005% tacrolimus eye drop for refractory vernal keratoconjunctivitis. Eye (Lond). 2011 Jul;25(7):872-80.
13. Sangwan VS, Jain V, Vemuganti GK, Murthy SI. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 2011 May;30(5):491-6.
14. Tseng S. Sutureless cryopreserved amnion grafts: prokera. The Ocular Surface Research and Education Foundation. Available at:
www.osref.org/media/documents/osref_prokera_instructionl_guide.pdf. Accessed January 2013.


