Since their introduction, contact lenses have been worn by millions of individuals, many of whom have had few or no problems with lens wear. Some wearers, however, have suffered complications ranging in severity from a mild inconvenience to loss of vision. But, contact lens complications are not a new phenomenon; in fact, a PubMed search of “contact lens complications” reveals two articles that were published as early as July 1960, discussing complications of contact lens wear. Since that time, over 2,100 peer-reviewed articles about contact lens complications that are unrelated to any form of infection have been published. In this article, we will focus on the pathophysiology, presentation, diagnosis and management of selected non-infections complications of contact lens wear.
Contact Lens-Related Eyelid Conditions
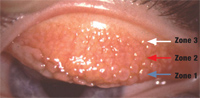
1. This patient presented with severe GPC after continuous (one month) wear of hydrogel lenses for several years. Zone 1 (blue arrow), Zone 2 (red arrow) and Zone 3 (white arrow) are involved.
The human blink rate varies with age and gender: in a 16-hour day, young males blink approximately 9,600 times, and young females blink roughly 15,000 times. But, these numbers increase with age—elderly females blink up to 22,000 times per day.1 Blinking, a dual-phase action, has both a downward and an upward movement. This is why in young males, the eyelid passes over the ocular surface or contact lens surface a minimum of 19,000 times per day. So, it should be no surprise to practitioners that contact lens wear impacts the eyelid.1
• Giant papillary conjunctivitis. In 1974, research found a papillary reaction on the upper tarsal conjunctiva in soft contact lenses wearers that closely resembled lid changes seen in ocular allergy.2 We now refer to this condition as giant papillary conjunctivitis (GPC) or contact lens–induced papillary conjunctivitis. In GPC, the upper tarsal conjunctiva undergoes changes beginning with nonspecific inflammation and progressing in severity to the appearance of characteristic papillary changes on the tarsal conjunctiva. Enlarged papillae (greater than 0.3mm in diameter) are a frequent finding in more advanced disease; they give the condition its name. These lesions may increase in size and number, and in some cases, they cover the entire tarsal surface (figures 1,2).3
Although soft contact lens wear, and to a lesser extent rigid lens wear are the predominant factors in the development of GPC, the condition has also been associated with other non-contact lens forms of conjunctival trauma. These include exposed suture barbs, ocular prosthesis and elevated filtering blebs, leading to the assertion that mechanical irritation is causative in GPC.4 Although GPC is more common in atopic individuals, and incidence of GPC peaks at the time seasonal allergies is most active, it is not a form of allergy.5 One study found coincident allergy in over 26% of individuals with GPC, and noted that they present with more severe signs and symptoms than non-allergic subjects.6
Individuals with GPC frequently report increased mucous discharge, blurred vision, ocular irritation and foreign body sensation. Contact lens wearers with GPC experience excessive lens movement, decreased lens tolerance and reduced wearing.7
Neutrophils and lymphocytes are normally only found in the epithelium and the substantia propria, while mast cells and plasma cells are present only in substantia propria. Basophils and eosinophils are rarely found in either layer. But in individuals with GPC, mast cells are found in the epithelium, and eosinophils and basophils populate both epithelium and substantia propria.8 Similarities between ocular allergy and GPC abound, but it is important to note the differences. Histamine levels are lower in GPC vs. allergic ocular states, perhaps explaining the relative infrequency of itching in GPC.8 The total conjunctival mass in GPC is twice that found in normal individuals, which is explained by the thickening of the conjunctiva and an increase in surface areas due to the numerous elevated papules. Biomicroscopic observation of papules is another strong indicator of the presence of GPC.9
Over the years, our understanding of the pathophysiology of contact lens-related GPC has advanced significantly. Coated contact lenses are a consistent finding in GPC, and lens coating increases as the condition worsens.8 Placing contact lenses from GPC patients onto the eyes of monkeys resulted in the development of GPC-like changes and a significant increase in tissue levels of IgE and IgG.10 In this study, contact lenses from patients who did not have GPC failed to elicit this response.10
As mentioned earlier, mechanical injury has also been implicated as a cause of GPC. Some believe that the coating in contact lenses acts as an antigen stimulus, causing production of tear immunoglobulins IgE, IgG, and in severe cases, IgM.8 The coating also causes mechanical trauma to the conjunctiva, resulting in the release of inflammatory mediators that attract eosinophils, mast cells, basophils and other leukocytes into the conjunctiva.
Another influence that plays a major role in the development of GPC is lens material. Hydrogel lenses typically cause a reaction that involves all three zones of the tarsal surface. Silicone hydrogel lenses usually cause a more focal papillary hypertrophy, occurring primarily in zones two and three.
Management of GPC is a multi-faceted task. It involves reducing or eliminating lens deposits and modulation of the inflammatory response. As was evidenced by one study, only 50% of GPC patients who switched to an improved cleaning regimen without replacing their daily wear lenses were able to continue lens wear. Lens replacement alone allowed 78% of patients to continue wearing their lenses. Ninety-four percent of patients who discontinued wearing contact lenses for three to four weeks and then were refitted with new lenses were able to continue wearing their lenses.6 Daily disposables are another option for individuals who have severe coating issues associated with GPC.
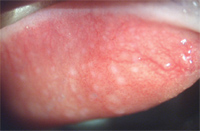
2. Focal areas of GPC in a silicone hydrogel lens wearer. The affected areas are concentrated in zones one and two.
GPC may be treated on a short-term basis with “soft steroids,” such as loteprednol etabonate, to quiet the inflammatory component of this condition. This drug does not, however, address the real underlying causes of GPC. So, in addition to pharmacotherapy, it is advisable to discontinue lens wear until the lid changes begin to regress.11 Topical mast cell stabilizers and combination antihistamine-mast cell stabilizers have also proved beneficial in treating GPC and do not carry the long-term risks associated with chronic corticosteroid therapy.8
• Meibomian gland dysfunction. Meibomian glands (tarsal glands) are holocrine glands located in the superior and inferior lids. These glands secrete meibum, a lipid composed primarily of non-polar lipids as well as polar lipids and small amounts of fatty acids.12,13 The presence of meibum on the surface of tears is critical to vision and comfort. The primary functions of the tear film lipid layer are to provide a smooth optical surface for refraction and to retard the evaporation of the water phase from the ocular surface.14 The longer a lens is worn, the more evaporation increases.15
A study examining the eyelids of contact lens wearers and non-wearers for morphologic changes in meibomian glands found shortening (condensation) of the meibomian glands in lens wearers that began distal to the orifices and gradually progressed toward the lid margins. After everting the upper and lower eyelids, the researchers photographed the meibomian glands with a noncontact infrared camera and an infrared filter. In several contact lens-wearing subjects, the length of the affected meibomian glands was less than half that observed in age matched control subjects. These changes were clinically significant in the upper lid but not in the lower lid of study subjects, suggesting that contact lens wear has a different effect on the lower lid vs. the upper.16 In an earlier study, the same group used infrared meibomography to evaluate the relationship of aging to meibomian gland morphology and found that the aging was linked to progressive loss of meibomian gland tissue.17 These findings suggest that contact lens wear may accelerate the age-related loss of meibomian glands, and that evaluation of meibomian gland structures would be advisable in contact lens wearers.16 The technique used in this study requires special infrared instrumentation; however, transillumination of the meibomian glands can be readily accomplished using a conventional transilluminator and slit lamp. The use of these tools permits the evaluation and detection of gland loss (figures 3,4).18
Contact Lens-Related Corneal Changes
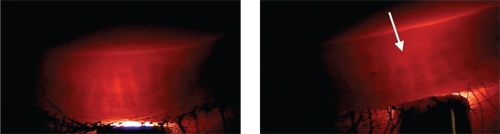
3. This biomicroscopic photo is of eyelid transillumination of normal meibomian glands.
4. Contact lens-associated meibomian gland loss is more common in the upper eyelids.
Cornea is a remarkable tissue! Under normal conditions, it is clear, structurally stable, resilient and resistant of infection and penetration. The intimate relationship between cornea and a contact lens can lead to multiple issues that affect lens comfort and vision.
• Contact lens acute red eye (CLARE). Patients who overwear their soft lenses or sleep in lenses that are not intended for overnight wear may awake early in the morning with symptoms of pain, photophobia and intense perilimbal injection and limbal edema or CLARE (figure 5). Focal or diffuse epithelial and sub-epithelial infiltrates are usually observed in the mid-peripheral cornea (figure 6). The etiology of CLARE is not completely understood, but several causes have been implicated; these include hypoxia, toxic effects from post-lens tear debris, mechanical irritation, dehydration of the tear lens during closed eye conditions, solution hypersensitivity and an immune response to toxicity or to bacterial toxins. Additionally, some propose that the hypoxic corneal epithelium produces a substance that leads to inflammation and leukocyte infiltration.19 The breakdown of cell membranes releases membrane phospholipids that can be converted into arachidonic acid. This is then converted by cyclooxygenase into prostaglandins and by lipoxygenase into leukotrienes. Pro-inflammatory prostaglandins promote inflammation, and leukotrienes are chemotactic for other leukocytes.20
Gram-negative bacteria in conjunction with contact lens overwear have also been implicated in the development of CLARE. There were two reported cases of CLARE in daily wear patients who wore their daily wear lenses on an extended basis. The lenses and case of the first patient cultured positive for Pseudomonas aeruginosa and Aeromonas hydrophila; the second patient cultured positive for Peudomonas aeruginosa and Serratia liquefaciens. Researchers propose that the release of endotoxins from the breakdown of the bacterial cell walls triggers the inflammatory response.21
Continuous wear of contact lenses may impact the metabolism and integrity of the cornea. Overnight wear of low Dk lenses results in hypoxia and hypercapnia (excess carbon dioxide). This may lead to excess lactate accumulation in the stroma, increasing osmolarity and causing metabolic acidosis, which results in corneal edema.22 In addition to the effects of corneal hypoxia, it appears that overnight soft lens wear with both high or low Dk lenses results in a significant increase of epithelial permeability.23
Treatment of CLARE is aimed at minimizing patient discomfort, recovery of normal function and patient education to prevent future occurrence. If patients have anterior chamber activity, they may benefit from cycloplegia during the initial 24 hours of management. In cases where there is significant or persistent cells and flare, topical steroids should be prescribed until the eye is quiet. Milder cases may be successfully managed with cycloplegia as well as topical and oral NSAIDs.24-26
Solution Hypersensitivity and Toxicity
5. This patient had worn her daily wear hydrogel lenses overnight and presented the following morning with CLARE—severe injection, infiltrates and pain.
Despite enormous advances in contact lens care solutions, solution-related issues continue to plague doctors and patients. Solution-related complications include type 1 immediate hypersensitivity (allergy), type 4 delayed hypersensitivity, toxic keratitis and conjunctivitis, corneal infiltration, and nonspecific irritation. Patients who present with solution-related problems can exhibit a wide variety of symptoms and signs.27 One study suggests that the highest rate of solution-related complications occurred in generic and private label products.28 These complications may also occur after patients use cleaners, storage solutions and drops that are inappropriate for their lenses.
When contact lens patients call with complaints of redness or irritation, staff members should always instruct them to bring in all of their solutions and drops to the examination. This becomes especially important when the patient has self-medicated with an over-the-counter (OTC) or a previously prescribed medication. Many prescription and OTC drops are not intended for use with contact lenses and contain preservatives that may become concentrated to a toxic level in soft contact lenses.29
Improper use of solutions may also lead to solution-related reactions. When patients fail to completely neutralize hydrogen peroxide-based solutions, or when they confuse their cleaning solution with their lens lubricating drops, they may develop a painful red eye with hazy vision secondary to corneal epithelial damage and edema, in part due to mitochondrial damage.30,31 Reviewing every product that the patient is using with his or her lenses is essential in thoroughly investigating the patient’s complaints.
Corneal Infiltrates and Ulcers
The presentation of white lesions in the central and mid-peripheral cornea of a contact lens wearer may represent a relatively benign or very ominous condition. The ultimate questions are: Is the lesion infectious or non-infectious, and is it an infiltrate or an ulcer? Baum and Dabezies examined nine contact lens patients who presented with mid-peripheral corneal infiltrates, all of whom had similar signs and symptoms. Working on the postulation that the lesions were sterile (non-infectious), they initially treated the patients with fluorometholone four times per day. After four days of treatment, eight of the nine subjects had rapid resolution of the infiltrates, but after one day of treatment, the remaining patient experienced increased pain and injection as well as decreased vision. Examination revealed enlargement of the infiltrate and overlying ulceration. Scrapings of the ulcer cultured positive for Pseudomonas aeruginosa, and the patient was treated with a topical antibiotic; her final visual acuity was 20/25. The author’s suggestions? When contact lens patients present with infiltrates, consider non-infectious causes, including extended wear, hypoxia, poor hygiene, case contamination, solution sensitivity and normal flora or bacterial toxins.32
Solution toxicity and hypersensitivity have been identified as a potential cause of corneal infiltrates. Hood evaluated patients who had previously been diagnosed with infiltrates attributed to solution hypersensitivity. The reported incidence of infiltrates was 0.7% for ReNu (Bausch + Lomb) and Opti-Free (Alcon), and 1% for AOSept (CIBA Vision). When these patients were rechallenged with the same solutions, the recurrence rate was 0% for AOSept, 0.3% for ReNu and 0.3% for Opti-Free.33 Based on his findings, the author suggested that many infiltrates attributed to solution reactions may actually be caused by bacteria or other agents.
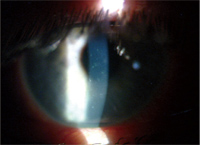
6. This photo shows the same individual seen in figure 5. Note the multiple
corneal infiltrates in the mid-peripheral and central cornea.
Hydrogen peroxide systems are a potential alternative in cases of hypersensitivity to multipurpose solutions. In fact, the lowest incidence of corneal infiltrative events was reported in silicone hydrogel wearers using hydrogen peroxide systems.34 Another study found that one-step hydrogen peroxide systems kill amoebic Acanthamoeba trophozoites, but are ineffective in killing cysts. While one-step systems may not adequately protect contact lens wearers from ocular amoebic infection, two-step systems are effective against both the cystic and trophozoites forms of Acanthamoeba.35
A prospective study looked at the risk for non-ulcerative contact lens complications in 877 contact lens wearers presenting to the emergency care department. The researchers found a lower rate of complications in patients wearing daily disposable vs. planned replacement lenses. Silicone hydrogel lenses wear was associated with a two-fold risk for “sterile keratitis.” Other factors that increased risk for non-infectious infiltrates included occasional and habitual overnight wear, less than 10 years of wear, poor hand hygiene and smoking.36 Silicone hydrogel lenses are increasingly popular for daily and overnight wear, and with good reason. But, despite their advantages, we should be aware that an increased risk for some non-infectious complications may be associated with their use.37
In addition to infectious and non-infectious ulceration of the cornea, autoimmune diseases can cause severe, focal inflammatory conditions, such as scleritis and peripheral ulcerative keratitis (PUK). In the early phase, PUK may mimic other forms of keratitis. The precise pathophysiology of PUK is not entirely understood, but it is thought to be mediated by T-cells and antibody-mediated infiltration. Collagenases and proteases secreted by neutrophils and macrophages cause destruction of peripheral corneal stroma.38 Patients present with pain and an excavated lesion—usually near the limbus—and in 36% of cases, coexistent scleritis is present, often in the same quadrant as the corneal disease. Peripheral ulcerative keratitis has been associated with immunologic disorders, such as Sjögren’s syndrome, Wegener’s granulomatosis, polyarteritis nodosa, Behset’s disease, relapsing polychondritis, or may also occur after ocular surgery.39,40 It is important to rule out PUK in the event that a patient with rheumatoid disease presents with an atypical corneal ulcer, especially if the individual is a contact lens wearer.40
Contact lens patients may present with a variety of conditions that mimic corneal ulcers or infiltrates. Many of these are not infectious and may be managed with topical steroid therapy. Barr proposed a scheme for initially differentiating between probable microbial keratitis and contact lens peripheral infiltrate (see, Differentiation of Corneal Lesions” above).41
If the provider treats a lesion as a sterile infiltrate and initiates steroid therapy, it is important to see the patient on the following day to assure that the infiltrate is not in fact an early ulcer. Should the condition deteriorate, consider other possible causes and modify therapy accordingly. In the case of any corneal infiltrate or ulcer that does not respond in the expected manner, always consider the possibility of herpes simplex keratitits.32
| Differentiation of Corneal Lesions
|
(Modified from Barr JT) |
| High Probability of Microbial Keratitis
1+ infiltrates >2mm in diameter Anterior chamber reaction or pain Mucopurulent discharge or positive culture |
High Probability Sterile Keratitis (CLPU or CLPI)
1+ infiltrates 0.75mm to 1mm diameter outside 6mm central zone Minimal or no anterior reaction or pain No mucopurulent discharge |
Corneal Neovascularization
The cornea is necessarily avascular; the presence of blood vessels in the cornea is a significant, and in some cases, a potentially sight-threatening condition. Corneal neovascularization is the formation of blood vessels in an area previously devoid of vessels (figure 7). Angiogenesis, the formation of new blood vessels from preexisting vascular structures, is found in tumor growth as well as in corneal and retinal disorders. A delicate balance exists between angiogenic factors that promote blood vessel growth (e.g., fibroblast growth factor and vascular endothelial growth factor) and anti-angiogenic molecules (e.g., angiostatin, endostatin or pigment epithelium derived factor) that inhibit vascularization of the cornea. Neovascularization occurs when upregulation of angiogenic factors occur, and new blood vessel growth begins.42 A number of inflammatory conditions and infectious disorders may precipitate corneal vascularization (CV), including autoimmune disease, viral, bacterial and fungal infections, as well as trauma and degenerative diseases.42 Using a murine model, corneas inoculated with keratitis manifested limbal capillary budding on the second day after inoculation. One day after the onset of C. albicans keratitis, VEGF-A was upregulated 12.5-fold. Corneal vessels grew toward the area of inflammation at the rate of 0.25±0.08mm/day and reached the central cornea on days six to seven.43 This study demonstrates the rapidity with which the process of neovascularization can be initiated and accomplished.
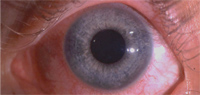
Contact lenses are a frequent cause of CV; 18% of extended soft lens wearers and 11% of daily wear soft lens patients show some degree of CV.44 The presumed cause of these changes is hypoxia. Early hypoxic changes are often seen as limbal hyperemia.45 Study subjects who wore low Dk lenses had a greater increase in limbal hyperemia. Low Dk patients experienced a three-fold increase in neovascularization, while subjects wearing high Dk lenses showed a slight decline in neovascularization and emptying of vessels previously encroaching on the cornea. Their findings suggests that hypoxic stress plays a major factor in the development of contact lens-related neovascularization.44
The management of contact lens-related neovascularization starts with discontinuation of lens wear and/or fitting the patient in a lens material of significantly higher Dk. The advent of silicone hydrogel lenses has provided a means of achieving that goal.45 Topical corticosteroids continue to be highly effective in suppressing actively proliferating corneal vessels; they are believed to exert anti-angiogenic effect through their anti-inflammatory properties (e.g., inhibition of chemotaxis and synthesis of pro-inflammatory cytokines). Prostaglandins are produced in angiogenesis; non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to inhibit this process. In cases that are recalcitrant to conventional therapy, laser has been an effective surgical alternative. Photodynamic therapy, a procedure popularized in the management of choroidal neovascularization has been used to treat corneal vascularization in animals. A photosensitizer is injected into systemic circulation or topically administered on the ocular surface, and a laser beam is directed at the affected area causing occlusion of the vessels.42 Currently, this technology is utilized to successfully treat human subjects as well.
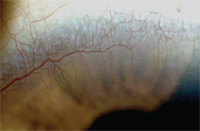
7. Corneal neovascularization in a highly myopic patient who intermittently wore her low Dk lenses overnight.
Corneal Limbal Stem Cell Deficiency
Stem cells are responsible for repopulating and maintaining the corneal epithelium. They reside exclusively in the limbal basal epithelium, the narrow zone between the cornea and the bulbar conjunctiva.47,48 Stem cells possess a unique subset of characteristics. They have a long life span, which may last as long as the natural life of the organism. But, stem cells are poorly differentiated, and their cytoplasm appears primitive compared to more differentiated cells. They also have a high capacity for self-renewal and a propensity for error-free proliferation and replication.48
Corneal limbal stem cell deficiency (CLSD) occurs secondary to chemical or thermal injuries, ultraviolet radiation, Stevens-Johnson syndrome, ocular cicatricial pemphigoid, ocular surgeries and/or cryotherapies, and severe microbial infection.48 Limbal stem cell deficiency has recently been implicated as a cause of ocular surface disease, such as pterygium. Limbal stem cells act as a barrier to the ingress of conjunctival cells onto the cornea. Limbal stem cell loss or deficiency negates or reduces this protective function and may lead to the replacement of corneal epithelium by conjunctival epithelium. This process, conjunctivalization, is characterized by surface irregularity, vascularization and the presence of goblet cells.49
The clinical presentation of CLSD includes persistent epithelial defects, superficial corneal vascularization, scarring and reduced visual acuity. Conjunctival epithelium lacks tight junctions and is therefore more permeable to fluorescein, so the affected surfaces show abnormal staining patterns (figures 8,9). These changes may be generalized or focal.49 Another important biomicroscopic finding that occurs in CLSD is the loss of the limbal palisades of Vogt.50
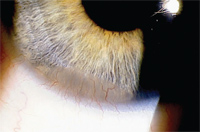
8. Conjunctivalization of the inferior cornea in a patient who chronically overwore his low DK lens and had poor hygiene.
Contact lenses have been identified as a cause of limbal stem cell loss.48,50 Martin conducted a study of patients with CLSD and found that the mean wearing time at diagnosis was 17.6 years (range six to 30). Seventy one percent of the individuals were asymptomatic at the time of diagnosis.50 He found that CLSD prevalent in contact lens wearers initially affects the superior conjunctiva, and as the process continues, conjunctival epithelium proliferates and eventually grows onto the cornea unless contact lens wear is discontinued.50 In addition to slit lamp findings, it is possible to corroborate the diagnosis of CLSD using impression cytology. The presence of goblet cells on the corneal surface is indicative of conjunctivalization of the cornea and indirectly confirms stem cell deficiency.51 Barbaro and coworkers report a new technique for using confocal microscopy and impression cytology to directly identify limbal stem cell loss.52
Contact lens-related CLSD is a serious and potentially sight threatening condition. In my practice, several young and middle-aged individuals have presented with a history of chronically wearing daily wear lenses that were not designated for extended wear (and inappropriate for extended wear based on Dk values). These patients presented with obvious signs and symptoms consistent with CLSD. To prevent further damage to the ocular surface, it is imperative that these individuals discontinue contact lens wear and be treated appropriately.
In less severe cases, CLSD can be managed with discontinuation of lens wear, a limited course topical steroid therapy and palliative measures. In cases of unilateral disease, small portions of donor tissue can be obtained from the non-affected eye and transplanted into the affected eye. This procedure is referred to as a limbal autograft.52,53
New evidence links contact lens wear to CLSD; this information should serve as a wake-up call to eye care practitioners who care for contact lens patients. We have yet another good reason to be careful observers of the limbus and cornea. Contact lens wear causes some degree of inflammation, and persistent inflammation has been recognized as a major threat leading to CLSD in humans. Any new neovasculariation, loss of the palisades of Vogt, or persistent keratitis in conjunction with these changes should prompt further investigation into abuse and overwear of low Dk lenses. It also gives us another reason to direct patients toward lens materials with higher oxygen permeability.
Recognize Complications and Treat Promptly
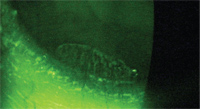
9. Fluorescein staining of the patient in figure 8 shows atypical staining of the conjunctivalized epithelium.
From their introduction, contact lenses have caused complications for some, but not all patients. It is not surprising—considering that we are placing a foreign object on the eye and leaving it there for hours, days or even weeks. Contact lens and solution manufacturers have developed safer and more comfortable products for our patients’ benefit. It is our charge to constantly watch for complications, whether they are symptomatic or not, and to be prepared to manage them before they have the chance to cause harm.
Dr. Townsend is in private practice in Canyon, Texas., and is an adjunct professor at the University of Houston College of Optometry. He is a member of the executive board of the Ocular Surface Society of Optometry (OSSO) a non-profit organization whose mission is to increase awareness and advance the understanding and management of dry eye and ocular surface disease.
1. Sforza C, Rango M, Galante D, et al. Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion. Ophthal Physiol Opt. 2008;28:345-53.
2. Spring TF. Reaction to hydrophilic lenses. Med J Aust. 1974;1(12):449-50.
3. Allansmith MR, Korb DR, Greiner JV, et al. Giant papillary conjunctivitis in contact lens wearers. Am J Ophthalmol. 1977;83(5):697-708.
4. Friedlaender MH. Some unusual nonallergic causes of giant papillary conjunctivitis. Trans Am Ophthalmol Soc. 1990;88:343-9.
5. Begley CG, Riggle A, Tuel JA. Association of giant papillary conjunctivitis with seasonal allergies. Optomi Vis Sci. 1990;67:192-5.
6. Donshik PC. Giant papillary conjunctivitis. Trans Am Ophthalmol Soc. 1994;92:687-744.
7. Abelson MB, Torkildsen G, Plumer A, et al. Giant Papillary Conjunctivitis; The dangers and treatment measures involved with “GPC.” RCCL. 2005;Nov;141(8):7.
8. Donshik PC, Ehlers WH, Ballow M. Giant papillary conjunctivitis. Immunol Allergy Clin North Am. 2008;28(1):83-103.
9. Greiner JV. Giant Papillary Conjunctivitis. In: Allergic Diseases of the Eye. Abelson MB (ed). New York: WB Saunders; 2001:140-60.
10. Ballow M, Donshik PC, Rapacz P, et al. Immune responses in monkeys to lenses from patients with contact lens-induced giant papillary conjunctivitis. CLAO J 1989;15(1):64-70.
11. Chin T. GPC: don’t call it an allergic reaction. Rev Optom. 2006 Nov;143(11).
12. McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003 Jul;1(3):97-106.
13. McCulley JP, Shine WE. Eyelid Ddsorders: The meibomian gland, blepharitis, and contact lenses. Eye & Contact Lens. 2003;29(1S): S93-5.
14. Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Experimental Eye Research. 2004;28:347-60.
15. Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004 Mar;78(3):389-94.
16. Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009 Mar;116(3):379-84.
17. Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomianglands in a normal population. Ophthalmology 2008;115:911-5.
18. Townsend WD. Eyelid Transillumination. Cont Lens Spect. 2007 Jan;22(1):23.
19. Zantos SG, Holden BA. Ocular changes associated with continuous wear of contact lenses. Aust J Optom. 1978;61:418.
20. Jaanus JE. Anti-inflammatory drugs in Bartlet JD, Jaanus SD. (eds). Clinical Ocular Pharmacology. Butterworth’s: Boston, 1989;182-5.
21. Sankaridurg PR, Vuppala N, Sreedharan A, et al. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol. 1996 Mar;44(1):29-32.
22. Bruce AS, Brennan NA. Corneal pathophysiology with contact lens wear. Surv Ophth. 1991:1:25-58.
23. Lin MC, Polse KA. Hypoxia, overnight wear, and tear stagnation effects on the corneal epithelium: data and proposed model. Eye & Contact Lens, 2007;33(6).
24. Dumbleton K. Adverse events with silicone hydrogel continuous wear. Contact Lens & Anterior Eye. 2002;25:137-46.
25. Mack C, Schaeffer J. Managing contact lens complications. Cont Lens Spect. 2004 Dec;19(12).
26. Townsend WD. Managing those rare contact lens complications. Cont Lens Spect. 1998 Dec;13(12).
27. Weisman BA. Assessing and treating complications. Cont Lens Spect. 2006 Jun;21(6).
28. Forister JF, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA Contact Lens Study. Eye & Contact Lens. 2009;4:176-80.
29. Chapman JM, Cheeks L, Green K. Interactions of benzalkonium chloride with soft and hard contact lenses. Arch Ophthalmol. 1990 Feb;108(2):244-6.
30. Memarzadeh F, Shamie N, Gaster RN, Chuck RS. Corneal and conjunctival toxicity from hydrogen peroxide: a patient with chronic self-induced injury. Ophthalmology. 2004 Aug;111(8):1546-9.
31. Atilano SR, Chwa M, Kim DW, et al. Hydrogen peroxide causes mitochondrial DNA damage in corneal epithelial cells. Cornea. 2009 May;28(4):426-33.
32. Baum J, Dabezies OH Jr. Pathogenesis and treatment of “sterile” midperipheral corneal infiltrates associated with soft contact lens use. Cornea. 2000 Nov;19(6):777-81.
33. Hood D. Do soft contact lens solutions cause corneal infiltrates? Contact Lens Spect. 1994 Feb;9(2):20-3.
34. Carnt NA, Evans VE, Naduvilath TJ, et al. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch Ophthalmol. 2009 Dec;127(12):1616-23.
35. Hiti K, Walochnik J, Faschinger C, et al. One- and two-step hydrogen peroxide contact lens disinfection solutions against Acanthamoeba: How effective are they? Eye. 2005;19:1301-5.
36. Radford CF, Minassian D, Dart JK, et al. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology. 2009 Mar;116(3):385-92.
37. Efron NA, Morgan PB. Trends in the use of silicone hydrogel contact lenses for daily wear. Contact Lens & Anterior Eye. 2008;31:242-3.
38. Galor A, Thorne JE. Scleritis and peripheral ulcerative keratitis. Rheum Dis Clin North Am. 2007 Nov; 33(4):835-4.
39. Silva BL, Cardozo JB, Marback P, et al. Peripheral ulcerative keratitis: a serious complication of rheumatoid arthritis. Rheumatol Int. 2010;30:1267-8.
40. Papaconstantinou D, Georgopoulos G, Kalantzis G, et al. Peripheral ulcerative keratitis after trabeculectomy in a patient with rheumatoid arthritis. Cornea. 2009 Jan;28(1):111-3.
41. Barr JT. 2004 Annual Report. Cont Lens Spect. 2006 Jan;20(1).
42. Chang J, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001 Aug;12(4):242-9.
43. Yuan X, Wilhelmus Kr. Corneal neovascularization during experimental fungal keratitis. Molecular Vision. 2009;15:1988-19.
44. Keech PM, Ichikawa L, Barlow W. A prospective study of contact lens complications in a managed care setting. Optom Vis Sci. 1996 Oct;73(10):653-8.
45. Papas E. Corneal vascularization and contact lenses. Arch Soc Espan Ofthalmol. 2006;81:309-12.
46. Hall RC, Barrett GD, Barry CJ, Constable IJ. Photodynamic therapy with verteporfin for corneal neovascularisation. Ophthalmic Surg Lasers Imaging. 2010 Mar;9:1-3.
47. Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010 Jul 8;363(2):147-55.
48. Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000 Mar-Apr;44(5):415-25.
49. Zaidi FH, Bloom PA, Corbett MC. Limbal stem cell deficiency: a clinical chameleon. Eye. 2003;17:837-9.
50. Martin R. Corneal conjunctivalisation in long-standing contact lens wearers. Clin Exp Optom. 2007;90:1:26-30.
51. Donisi PM, Rama P, Fasolo A, Ponzin D. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003 Aug;22(6):533-8.
52. Barbaro V, Ferrari S, Fasolo A, et al. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol. 2010 Jul;94(7):926-32.
53. Kheirkhah A, Raju VK, Tseng SCG. Minimal Conjunctival Limbal Autograft for Total Limbal Stem Cell Deficiency. Cornea. 2008 July; 27(6):730-3.


