While traditional penetrating keratoplasty is successful in treating many corneal diseases to prevent blindness, sometimes standard corneal transplants fail. When this happens, a keratoprosthesis is an important alternative treatment modality. Furthermore, for the pediatric population and other high-risk conditions such as chemical burns, ocular trauma, herpetic keratitis, Stevens-Johnson syndrome and mucous membrane pemphigoid, researchers have increasingly advocated it as a primary penetrating corneal procedure over standard penetrating keratoplasty.1-3
| One or Two? 1. Palioura S, Chodosh J. Boston keratoprosthesis type II: indications, techniques, outcomes, and management. In: Cortina M, de la Cruz J, eds. Keratoprostheses and Artificial Corneas. Berlin: Springer; 2015:69-179. |
The basic principle of keratoprostheses is the insertion of a clear “window” into the ocular surface to maintain a clear visual axis, independent of the state of the surrounding cornea. Numerous materials with favorable optical properties are used for this purpose, as are diverse strategies for biointegration into the surrounding ocular surface tissues. Thus, patients can accomplish a prompt recovery of best achievable visual acuity and good stability of the implant.
Most advances in the field of keratoprosthesis research have occurred during the second half of the twentieth century, as recognition of the limitations of penetrating keratoplasty, an inadequate supply of donor tissue and the development of new synthetic polymers sparked increased interest in keratoprostheses.2,4-7 This article focuses on the most widely used keratoprosthesis in the United States, the Boston keratoprosthesis type 1 (KPro).
Boston KPro Basics
This keratoprosthesis was developed by Claes Dohlman, MD, PhD, at the Massachusetts Eye and Ear Infirmary in 1968.4,8,9 Dr. Dohlman and colleagues have introduced many improvements to the original collar button design over the past five decades.9,10 The device obtained FDA clearance in 1992, and more than 12,000 of these devices have been implanted worldwide.5,8
Two types of Boston keratoprostheses exist, both consisting of two plates joined by a polymethyl methacrylate (PMMA) stem, which is the optical portion. They are designed to be incorporated into a ring-shaped cadaver corneal graft, which can be donor corneal tissue not suitable for endothelial replacement, since healthy endothelium is not required.
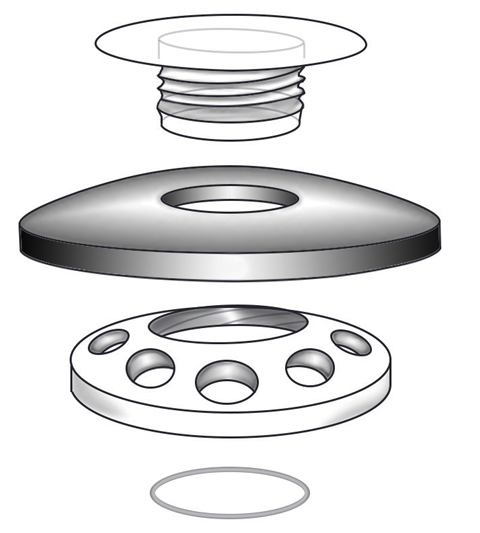 |
| This schematic drawing illustrates the anterior to posterior assembly of a Boston KPro type 1. |
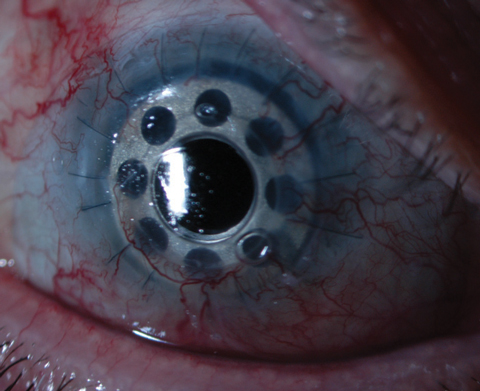
Design
The Boston KPro type 1 has a PMMA front plate that is 5.5mm in diameter, into which the appropriate dioptric power has been ground.4 The front plate is fused to a 3.35mm cylindrical optical stem, onto which the surgeon slides the different assembly components. The PMMA back plate is 8.5mm in diameter and has 16 fenestrations, each 1.3mm in diameter. The fenestrated back plate is important for nutrient exchange with graft keratocytes from the aqueous and to keep the corneal graft hydrated. Finally, a locking ring, either titanium or PMMA, is placed behind the back plate to secure the assembly.
New research suggests titanium may be superior to PMMA for the back plate.6,11 The Boston KPro with a titanium back plate now has FDA approval and is largely replacing the older PMMA version, but both material options remain available and marketed under the same name, the Boston KPro type 1. Not only is titanium superior from a manufacturing and resilience standpoint, but as an inert material it also appears to cause less postoperative inflammation and retroprosthesis membrane formation.6
Patient Selection
The Boston KPro type 1 is best suited in cases of refractory corneal blindness with repeated graft failure and pediatric congenital corneal opacities, such as Peter’s anomaly.1-3,12
Blink rate and tear production are important patient selection factors because this device often fails in the setting of a dry ocular surface.5,6,13 Therefore, clinicians should perform a Schirmer’s test, note lagophthalmos or other lid pathology and estimate the rate and completeness of blinking preoperatively (when the patient is not aware they are being observed) before referring a patient for a keratoprosthesis. Practitioners should also inspect the conjunctiva for any evidence of inflammation, surface keratinization and symblephara, which may need to be addressed concurrently.
The Boston KPro type 1 can be successful in patients with cicatrizing diseases (e.g., Stevens-Johnson syndrome, mucous membrane pemphigoid, chemical burns) when there is a good blink and tear film.5,6,13 However, these groups of patients remain a high-risk category and a type 2 Boston KPro is often considered in these conditions.
Procedure
The surgery for a Boston KPro type 1 implantation is analogous to regular penetrating keratoplasty. Either general or retrobulbar anesthesia can be used. Surgical steps include:
1. The surgeon slides an 8.5mm corneal donor button—in which a central 3mm hole has been punched—onto the stem of the front plate.
2. The titanium or PMMA back plate is then advanced onto the cylindrical stem to sandwich the donor graft between the two plates.
3. The surgeon slides the titanium ring onto the stem and snaps it into place, securing the entire assembly. The patient’s cornea is then trephined in the standard manner.
4. The donor button/keratoprosthesis complex is sutured into the patient’s cornea, as in a standard penetrating keratoplasty, using nylon interrupted sutures.
5. At the end of the procedure, the surgeon places a 16.0mm diameter, 9.8mm base curve, plano soft Kontur contact lens onto the eye as a bandage lens.
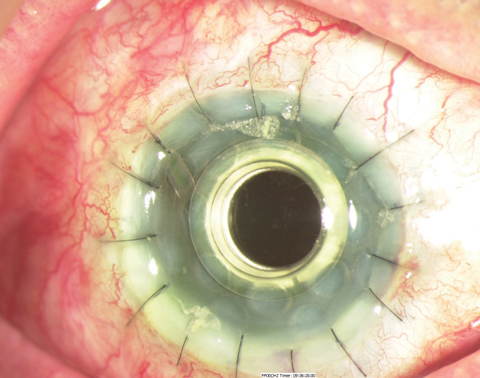 |
| This is a Boston KPro type 1 in the early postoperative period. |
Post-op Care
Postoperatively, patients are started on prednisolone acetate 1% QID for one week and tapered over a period of two to three months. A slower taper is advised if one notes a fast rate of a retroprosthetic membrane formation. However, a prolonged taper may increase the risk of a fungal infection, especially in endemic areas. Patients are also started on topical polymyxin B/trimethoprim QID for one week, then maintained on a once daily prophylactic dose. Vancomycin (14 mg/mL) at the same frequency is additionally recommended in patients with chemical burns or autoimmune conditions. Alternatively, a combination of a fourth-generation fluoroquinolone and vancomycin can be used.
Outcomes
When implanted in low-risk eyes, the Boston KPro type 1 has a high retention rate—greater than 80% beyond 40 months, according to two recent studies.14,15 In a prospective cohort study, 300 patients demonstrated a retention rate of 93% after an average of 17 months.16 The preoperative mean acuity was 20/1205, and final mean acuity was 20/150.16
In another prospective multicentered study, 60% of 133 patients achieved a best-corrected visual acuity of 20/200 or better, which was retained in 90% of eyes during the reported follow-up of 8.5 months.17 Multiple small studies support these numbers, and many show improved outcomes with the newer device designs.14,15
The prognosis for high-risk patients, such as those with severe autoimmune disease or chemical burns, is more guarded with variable results. Retention rates generally are lower, ranging from 59% to 89%, with a visual acuity of 20/200 in approximately two-thirds of cases.6,14-18
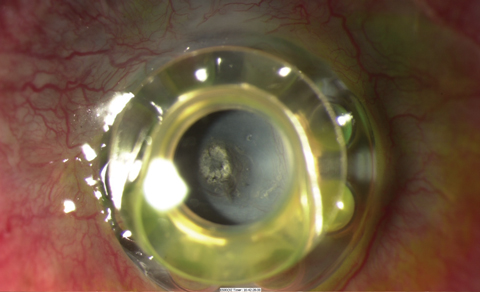 |
| A dense retroprosthetic membrane in a Boston KPro type 1, as seen here, required surgical removal, but Nd:YAG laser was attempted in the early stage of membrane formation. This patient also has extrusion with the front plate vaulting from the ocular surface. |
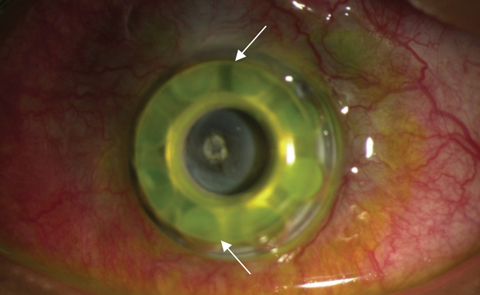 |
| The white arrows in this lower magnification view of the same patient delineate the edge of the front plate. This patient has extrusion with the front plate vaulting from the ocular surface. The donor corneal graft is no longer visible after corneal melt. |
Complications
Several possible concerns exist for this procedure, including:
Retroprosthetic membranes. These are commonly reported from 13% to 60%, and likely result from intraocular inflammation.17,19,20 Histopathologic studies with the Boston KPro show that the membranes originate from the host’s corneal stroma.5,6,11 Luckily, the development of the titanium back plate has reduced their incidence.6,11 Peribulbar steroid injections may be useful in the early stages of membrane formation to slow progression.
Once the membrane has formed, an Nd:YAG laser, followed by topical steroids, can create an opening in the membrane and restore vision. If the membrane becomes too thick and vascularized, surgical management becomes necessary.
Melts and extrusion. Corneal melts tend to occur at the edge of the front plate. Biomicroscopy and anterior segment optical coherence tomography are helpful in detecting and monitoring corneal thinning around the keratoprosthesis. A leak on Seidel testing should prompt an ultrasound to check for choroidal effusions. Kissing choroidal detachments frequently lead to retinal detachment and have a very poor prognosis for visual recovery.
Research shows a good bandage soft contact lens fit and retention reduces the risk for corneal melts and extrusion.21 Also, a shortened use of topical corticosteroids and avoiding toxic drops, such as fortified vancomycin, are helpful in reducing the risk of corneal melts.
Infectious endophthalmitis. This remains the most common posterior segment complication following keratoprosthesis surgery.5,14-16,22 It usually presents following microbial keratitis with decreased vision in an injected and painful eye, and often shows a leak on Seidel testing. Prophylactic topical antibiotics have greatly reduced bacterial endophthalmitis rates, with recent studies reporting 0% to 14.7%.5,14-16,22-25 Treatment generally consists of leak repair, intravitreal tap and antibiotic injection, and use of topical antibiotics.
While wearing an extended wear contact lens, fungal infections can also arise with chronic treatment involving corticosteroids and antibiotics. In one series, four out of 202 eyes with a keratoprosthesis developed a confirmed fungal keratitis or endophthalmitis over a cumulative 6,893 patient-months of follow-up.26 If clinicians see fungal colonization of the contact lens, they should change the contact lens and administer a course of topical amphotericin B 0.15%. The prognosis in fungal infections is usually good if identified early.
Another manifestation of ocular inflammation is a sterile uveitis and vitritis. This condition masquerades as a bacterial endophthalmitis with a reduction in vision; however, no pain, tenderness or redness is typically observed. Treatment with topical and peribulbar steroids typically results in restoration of baseline vision.
 |
| This KPro patient was fit with a custom bandage lens because the standard KPro bandage lens would not stay on eye. Photo: Clark Chang, OD, MSA, MSc |
Glaucoma. This is currently the single most serious complication following Boston KPro surgery. Although many patients have glaucoma preoperatively, it frequently worsens in some and develops in others following keratoprosthesis surgery.5,14-16,22-25 Research suggests chronic low-grade inflammation, progressive angle closure and anterior displacement of the iris are all factors in glaucoma progression.
Intraocular pressure (IOP) is the most important risk factor for developing glaucoma, yet it cannot be reliably measured in Boston KPro patients, even though clinicians should estimate IOP with finger palpation at every visit. The lack of objectivity of such a measurement has prompted the development of alternative methods such as scleral tonometry, which has been tried with variable success.27,28
Patients with glaucoma or suspicion should be managed in conjunction with a specialist. Often, surgeons consider implanting a glaucoma drainage device concomitantly during surgery.
Contact lens-related complications. Soft contact lenses have become the standard of care to protect against evaporative damage to the corneal surface around the Boston KPro. They can be worn for many months and even years without replacement. Extended contact lens wear is actually recommended, and the lens helps to protect a fragile ocular surface from mechanical stress and desiccation, reducing the risk of stromal thinning and corneal melt.21 Although uncommon, extreme lens deposits can reduce visual acuity. This seems to be more related to a low blink rate and incomplete blinking, rather than low Schirmer values. Refitting into a hybrid or large diameter rigid gas permeable lens can be beneficial for reducing lens deposits.
The Boston keratoprosthesis type 1 is now considered a viable alternative to standard corneal transplantation for specific corneal conditions. Careful patient selection is paramount, since these patients will require lifelong monitoring, often from multiple subspecialists. Notwithstanding, there is no question that the Boston KPro has helped restore vision in many blinded by severe corneal disease.
Dr. Esposito is an attending provider and clinical researcher at the New Mexico Veterans Administration Health Care System eye clinic in Albuquerque, New Mexico. He is an adjunct clinical professor and faculty at University of Houston College of Optometry, Pacific University College of Optometry and New England College of Optometry.
1. Aquavella JV, Gearinger MD, Akpek EK, McCormick GJ. Pediatric keratoprosthesis. Ophthalmology. 2007;114:989–94. |


