A Deeper Look at DEWS II The Tear Film: More Complex Than You May Realize |
In 2007, the Tear Film and Ocular Surface Society’s Dry Eye Workshop (TFOS DEWS) triggered widespread recognition of dry eye disease (DED), as well as appreciation for its debilitating impact on quality of life and growing prevalence worldwide.1 In addition to providing the first international consensus summary of the existing literature at the time, the report provided recommendations for future trials and studies. In the 10 years following that initial report, the total number of publications relating to DED almost doubled.2 The newfound enthusiasm and interest has now spread beyond academia to clinical and industrial sectors, providing aid to the millions with dry eye across the globe.
This past July, the second DEWS report (DEWS II) was published.2 This international collaborative initiative reviewed the thousands of scientific articles published in the field since the original workshop and involved over 150 clinical and basic research experts from 23 countries. The workshop was divided into 12 subcommittees in an effort to generate a consensus understanding of the major aspects of DED. The resulting reports have redefined and reclassified DED, exploring everything from its diagnosis and treatment to recommendations for future clinical trials.3-12 Here, we provide an overview of the DEWS II Definition and Classification and Diagnostic Methodology reports, and describe how each can be used to help patients in clinical practice.3,9
 |
| Fig. 1. One important takeaway from the new classification system is its emphasis on mixed forms of dry eye, which is more prevalent than was previously known, according to TFOS DEWS II. Adapted and reprinted from Ocular Surface (2017) 276–283, Craig JP, Nichols KK, Nichols JJ, et al. TFOS DEWS II definition and classification report, p. 281, © 2017, with permission from Elsevier. |
Definition and Classification
The TFOS DEWS II Definition and Classification subcommittee redefines dry eye as “a multifactorial disease of the ocular surface characterised by the loss of homeostasis, and accompanied by ocular symptoms, in which tear film instability, hyperosmolarity, inflammation, and ocular surface damage, and neurosensory abnormalities, play etiological roles.”3
Consistent with the original DEWS definition, the multifactorial nature of dry eye is acknowledged in the DEWS II definition.1 Furthermore, its status as a disease is recognized in order to reflect its significant impact on ocular surface tissues and patient quality of life. New components of the definition include acknowledgement of DED’s association with a “loss of homeostasis.” This encompasses the diverse range of signs that are possible with ocular surface and tear film imbalance. Also, the phrase “accompanied by ocular symptoms” has been broadened to include symptoms that extend beyond those of ophthalmic discomfort, dryness and visual disturbance. Hence, DED diagnosis requires the presence of both clinical signs and symptoms. Finally, to allow for differentiation of DED from other forms of ocular surface disease (OSD), the key etiological drivers that predispose patients to the self-perpetuating vicious circle of DED have also been included in the definition.4
 |
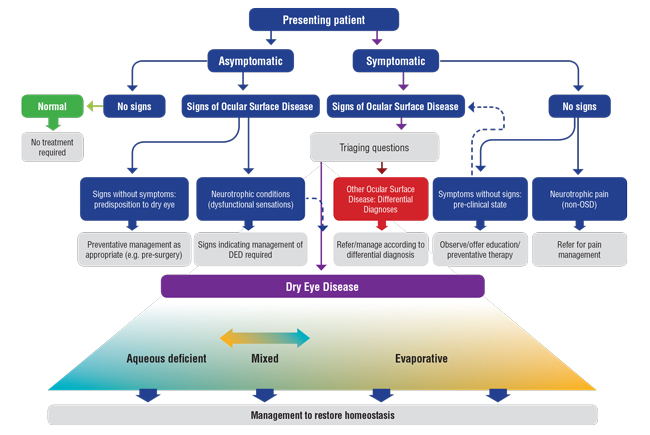
| Fig. 2. Signs of blepharitis and low lacrimal lake noted prior to cataract surgery. |
The classification of DED is particularly important in clinical practice, allowing eye care providers to understand where a patient fits within the context of OSD, and thereby tailor dry eye management and treatment strategies accordingly (Figure 1).3,11 While DEWS II has brought about a patient-centered approach to facilitate the diagnosis of DED patients who exhibit both clinically detectable signs and symptoms, it also acknowledges patients who present with either signs but not symptoms or symptoms but not signs. Without this distinction, the previous DEWS report risked the potential for inadequate management strategies and unrealistic expectations. In studies and clinical trials, inappropriate inclusion of patients without observable dry eye signs or symptoms could also subject new therapies and drugs to unachievable efficacy thresholds during the regulatory approval process, given the difficulty in detecting improvements in clinical signs and symptoms should one or the other fail to exist at the outset.
One example of a case with objective signs, but no subjective symptoms, would be a patient with clinical features of OSD detected incidentally during routine ophthalmic examination, such as before a contact lens fitting or before an operation (Figure 2). Another instance would be a patient with reduced corneal sensation, which may arise from neurotrophic conditions. While the treatment for these patients will involve dry eye therapies, differentiating these individuals from those exhibiting both clinical signs and symptoms helps facilitate the development of management plans and targets that acknowledge these differences in baseline status.
The classification system also accounts for patients with symptoms that appear to be incongruent with the signs identified from objective ophthalmic examination. These cases could indicate an early pre-clinical stage, which may require careful monitoring for progression into DED in combination with patient education. However, they may also potentially reflect an element of neuropathic pain, which can be a result of peripheral or central nervous system sensitization.6 When the ocular surface is not driving the pain reported by the patient, topical treatments are unlikely to provide relief. If this is the case, a referral to a chronic pain clinic may be warranted, as neurosensory abnormalities typically fall outside the scope of conventional therapeutic strategies for DED.
As in prior classification systems, aqueous deficient dry eye and evaporative dry eye are the major subtypes of DED in DEWS II. However, greater emphasis has been placed on the overlap between the two subtypes, given their complex inter-relationship in the vicious circle perpetuating DED. Regardless of etiology, the central mechanism of DED involves hyper-evaporation of the tear film, which results in hyperosmolarity.4
In evaporative dry eye (most commonly the result of meibomian gland dysfunction [MGD]), deficiency in the tear film lipid layer compromises its ability to inhibit tear evaporation, thereby causing hyperosmolarity. It is also thought that other factors, such as incomplete blinking, may have an additive effect on evaporative losses from the tear film. In aqueous deficient dry eye, rapid breakup and associated instability of the tear film may exacerbate its evaporative mechanisms.
Aqueous deficient dry eye has multiple causes, including inflammatory infiltration of the lacrimal gland, acinar and ductal epithelial dysfunction. However, blockage of the lacrimal gland’s sensory drive and use of systemic drugs including anti-histamines, beta-blockers and diuretics are also recognized causes.4
The tear film hyperosmolarity that occurs in both dry eye subtypes can trigger multiple ocular surface inflammation cascades and pathways. The release of inflammatory mediators and proteases can lead to the loss of epithelial and goblet cells, as well as damage to the epithelial glycocalyx. The resulting tear film instability further exacerbates the pre-existing hyperosmolarity, ultimately leading the vicious circle of DED to damage all components of the ocular surface and tear film.4
 |
| Fig. 3. The TFOS DEWS II diagnostic algorithm prioritizes the role of triaging questions in a dry eye work-up. Adapted and reprinted from Ocular Surface (2017) 544–579, Wolffsohn JS, Arita R, Chalmers, R, et al. TFOS DEWS II diagnostic methodology report, p. 561, © 2017, with permission from Elsevier. |
Diagnostic Methodology
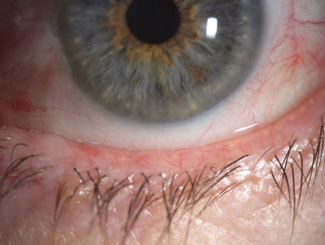
In the past, reported DED prevalence rates have varied considerably, partly reflecting the heterogeneity in diagnostic criteria applied in the different studies conducted around the world.7 Consequently, the DEWS II Diagnostic Methodology subcommittee sought to develop a consistent set of diagnostic criteria for DED to be used in future studies.9 Their report involved a thorough review of the diagnostic accuracy data of validated symptomology questionnaires, as well as ocular surface and tear film measurements from the published literature. This led to a consensus diagnostic battery of tests for DED (Figure 3).
 |
| Fig. 4. Mires reflected from the tear film surface allow measurement of tear stability noninvasively. |
In their consensus report, the subcommittee acknowledged the need for clinicians and researchers to differentiate DED from other ocular surface conditions that may confound the diagnosis of DED.
To address this, they developed a series of triaging questions to facilitate the identification of differential diagnoses and comorbidities that may require treatment prior to the management of any residual DED. The triaging questions, listed in Figure 3, are designed to be used before the diagnostic battery of tests to encourage differentiation of conditions such as ocular infection and allergy from DED. Any positive responses to the questions should be explored in depth and supplemented with evaluation of relevant clinical signs.
In accordance with the revised DED definition, symptoms and at least one sign of disrupted tear film homoeostasis must be present to reach a dry eye diagnosis.3 To aid in this process, the subcommittee selected the five-item Dry Eye Questionnaire (DEQ-5) and the Ocular Surface Disease Index (OSDI) as the validated questionnaires of choice in assessing the existence of dry eye symptomology.9 A positive symptom score requires a diagnostic cut-off score of either ≥6 on the DEQ-5 or ≥13 on the OSDI.
If the patient meets the positive requirement for dry eye symptoms, evaluation should be conducted to confirm whether there are signs of disruption to the tear film or ocular surface homeostasis. This can be confirmed by positive scores in any one of the following three measurements: tear film stability, tear osmolarity or ocular surface staining.
Whenever possible, tear film stability should be measured using noninvasive methods that reflect mires from the surface of the tear film (Figure 4). A diagnostic cut-off break-up time of <10s measured by subjective observation is indicative of tear film instability.
 |
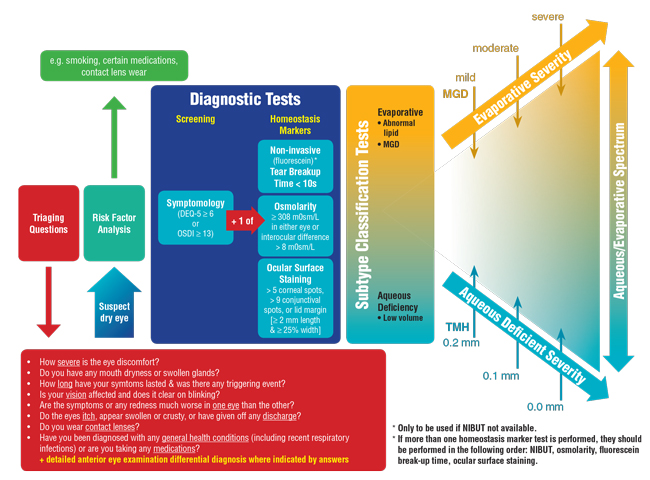
| Fig. 5. Lid wiper staining with lissamine green indicates ocular surface damage. |
Aqueous sodium fluorescein instillation is no longer recommended for assessing tear film stability because it is associated with tear film destabilization, which artificially decreases breakup time measurements. A minimal amount of fluorescein can be applied via a wetted strip, but only if a noninvasive method is unavailable. In these cases, excess fluid must be shaken off the strip before applying it to the temporal canthal region, and the fluorescein break-up time measurement should be recorded with the aid of a yellow barrier filter to optimize visualization.
In clinical settings, tear osmolarity is commonly measured with the TearLab osmolarity test. With this instrument, a diagnostic cut-off value of ≥308mOsmol/L in the eye with the higher osmolarity, or an interocular difference in osmolarity of >8mOsmol/L constitutes an ocular surface homeostasis disruption.
Ocular surface staining with both sodium fluorescein and lissamine green dyes is assessed under slit lamp biomicroscopy, again with a yellow filter, to optimize visualization of fluorescein. Positive staining is indicated by any one of the following: >5 spots within the cornea, >9 spots in the conjunctiva or lid wiper epitheliopathy that extends at least 2mm along the length of the lid margin and at least 25% of the width (Figure 5).
Should DED be confirmed, it is important to determine the relative contribution of aqueous deficiency and hyper-evaporative mechanisms to appropriately tailor therapeutic choices the needs of the individual patient. Tear meniscus height measurement is the preferred noninvasive diagnostic test for aqueous deficiency, with a value of <0.2mm suggesting a tear-deficient state. While other tests such as the phenol red thread test and the Schirmer’s test also offer an indirect measure of tear volume, they are not recommended because of their invasive nature. Measurements recorded under topical anesthesia are not suitable replacements, as they are neither reliable nor repeatable.9
MGD is considered the leading cause of evaporative dry eye, and therefore gland morphology and function warrants careful evaluation.14-17 Infrared meibography can be used to assess morphology, and gland function can be directly assessed with the Meibomian Gland Evaluator (TearScience) diagnostic expression or by applying gentle digital pressure. Meibomian gland function can also be indirectly assessed by looking at the quality of lipid layer patterns interferometrically, either with qualitative instruments or with quantitative instruments.
With the revised dry eye definition and classification scheme, as well as the newly developed diagnostic criteria, the hope is that clinicians will be able to better tailor management plans towards the unique needs of their patients and set realistic treatment expectations. Additionally, these guidelines may assist researchers in defining enrollment criteria for participant recruitment in clinical studies and serve as a useful reference for regulators involved in the approval of novel dry eye therapies.
Dr. Craig is an associate professor of ophthalmology at the University of Auckland. She was a co-chair of the TFOS DEWS II Definition and Classification subcommittee.
Dr. Wolffsohn is an associate pro-vice chancellor and professor of optometry at Aston University. He was a chair of the the TFOS DEWS II Diagnostic Methodology subcommittee
Mr. Wang is a doctoral student in ophthalmology at the University of Auckland and a foundation year house officer at Auckland Hospital.
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92. |


