Scleral lens use has increased exponentially in the last decade—beyond being indicated for irregular corneas —to a broad range of contact lens patients, including those with normal corneal curvature.1 However, though these specialty lenses are beneficial and even life-changing in some cases, their unique fitting characteristics mean there are also inherent challenges that make lens wear more difficult. This article will discuss several concepts practitioners should remain aware of when fitting scleral lenses.
Removing the Cloud
Non-wetting lenses and post-lens tear debris are two relatively common occurrences during scleral lens wear that can result in suboptimal or “cloudy” vision.2 Mucins and lipids are attracted to the hydrophobic oxygen permeable scleral lens material, forming deposits and resulting in poor wettability. Research has shown that post-lens tear debris consists of a high concentration of lipids; unsurprisingly, lens surface and tear reservoir clouding issues are common in patients with ocular surface disease.3
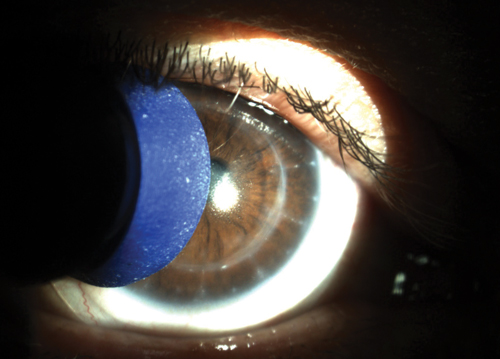 | |
| Fig. 1. A plunger soaked in gas permeable contact lens conditioner can be used to gently scrub away lens surface deposits and improve wettability. |
In the case of a non-wetting lens, the first step is to aggressively treat the patient’s ocular surface disease. Despite changing the lens design and material, the surface of the lens itself may remain compromised if the condition isn’t appropriately managed.
Don’t forget to look for the presence of Demodex as well: these mites infest the eyelash follicle and/or meibomian glands, and often trigger the accumulation of cylindrical dandruff around the base of the eyelashes.4 Infestations can lead to inflammation of the meibomian glands and subsequent meibomian gland dysfunction (MGD), disruption of the tear film and a non-wetting lens. However, conventional MGD treatments like warm compresses and lid hygiene are often ineffective in treating the infestation; instead, tea tree oil in the form of wipes or eyelid scrubs should be considered. Keeping this condition in mind and treating it appropriately can help reduce its effects on a non-wetting lens.
Selecting a material with a low wetting angle can help improve the wettability of the lens and reduce the hydrophobic areas that attract deposits. Plasma treatments can also help achieve sufficient wettability. Recommend that patients wash their hands with non-oily soaps such as those made for contact lens wearers or acne sufferers. Oils in many soaps can leave a residue on the lenses that may affect lens wetting. Optimum by Lobob’s Contact Lens Wearer Hand Soap and Ocusoft’s hand Soap are good examples; neither contains oils that can transfer from the patient’s hands to the contact lens. Similarly, residue left on plungers used for insertion can also compromise the lens surface upon contact. Instead of disinfecting the face of the plunger with alcohol, which can further compromise the hydrophilic surface, soak it in gas permeable contact lens conditioner to remove residue. Frequent replacement of plungers is also beneficial. For patients who wear makeup or face cream, recommend they use oil-free products and apply them following lens insertion to prevent debris or residue from affecting the lens.
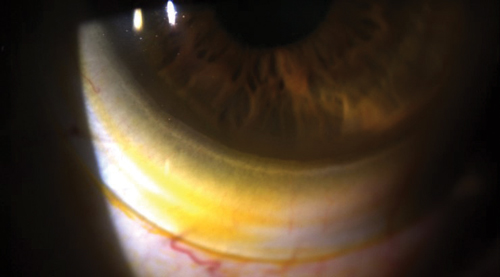 | |
| Fig. 2. Sodium fluorescein tear exchange at the edge of a scleral lens. | |
 | |
| Fig. 3. Overfill the lens bowl with nonpreserved saline to prevent application bubbles. |
Use a cotton swab soaked in gas permeable contact lens conditioner or nonpreserved saline to wipe away any deposits while rewetting the lens surface. Conditioner can also be placed on a plunger to gently scrub the front lens surface much like a squeegee (Figure 1). Cleaning the lens with a two-component cleaner of sodium hypochlorite and potassium bromide (Progent, Menicon) for 30 minutes once a week or biweekly can help with eliminating surface deposits. Miraflow, an alcohol-based cleaner can be used for 30 seconds to remove additional deposits.
Use of artificial tears containing a more viscous agent like nonpreserved carmellose sodium (Celluvisc, Allergan) or carboxymethylcellulose sodium (Optive, Allergan or Theratears) in place of saline to fill the lens bowl can act as an obstacle to prevent debris from moving through and underneath the lens. Decreasing sagittal depth or changing scleral lens designs is beneficial to reduce debris. In addition, achieving optimal peripheral lens alignment can also reduce the chance of debris traveling underneath the lens. This can be accomplished by moving to a smaller diameter scleral lens or use of toric peripheral curves. Squirting nonpreserved saline parallel to the edge of the lens can rinse off debris from underneath; patients can also remove and reinsert the scleral lens with fresh nonpreserved saline mid-day.
While non-wetting lenses and post-lens tear debris can result in suboptimal or “cloudy” vision, reduced vision can arise as result of contact lens flexure. Flexure can be managed by increasing the center thickness, flattening the base curve radius, or changing to a stiffer material (typically from a higher to a lower oxygen permeability [Dk]).5 Prior to making these lens changes, it is important to verify that the reduced vision is due to flexure instead of true uncorrected astigmatism. Flexure can be diagnosed by performing keratometry over the patient’s scleral lenses.5 Front surface toric scleral lens options are available for patients who have true residual astigmatism.
Derailing Discomfort
Patients can report lens awareness for several reasons, including the presence of air bubbles, excessive edge lift, a tight lens or corneal touch. Check for tear exchange by dabbing sodium fluorescein on the bulbar conjunctiva (Figure 2). If the dye cannot be detected underneath the lens, its peripheral curves should be flattened since the lens is likely too tight. Conversely, if excessive edge lift is the problem, rectify the situation by steepening the peripheral curves.
Air bubbles are the result of spillage of the filling solution during lens application or insufficient filling of the bowl of the lens. If bubbles are an issue, re-educate on proper application technique, including filling the lens bowl adequately. Additionally, suggest consideration of a more viscous agent such as Celluvisc to fill the lens bowl (Figure 3).
Useful tools that can assist patients with lens application include the See Green Lens Inserter (Dalsey Adaptives), which is available with and without a stand. The stand holds the plunger and lens in place prior to application. This is especially helpful for patients who have unsteady hands or for those who require both hands to hold their eyelids open. A second tool, the EZi Scleral Lens Applicator (Q-Case), is placed on the finger like a ring, with a base for scleral lens application. This design provides stability and allows patients to apply scleral devices with one finger. No. 8 O-rings can be obtained at the hardware store with dimensions measuring 3/8” x 9/16” x 3/32.” These hold the scleral lens on the patient’s finger to allow for stable application. Alternatively, single-use sterile orthodontic rings placed on the patient’s hand can be used for lens insertion. These are available in packages of 100.
Check for corneal touch after three to four hours of lens wear, as corneal touch may be missed during the initial visit. If corneal touch is noted, increase the sagittal depth of the lens. For practitioners new to fitting scleral lenses, try first evaluating the lens at the lens dispense visit and then again at four hours after the initial lens dispense. This additional visit is helpful to determine if any corneal touch is present. If so, reorder the lens prior to dispensing for further wear.
In some cases, attempt piggybacking with a silicone hydrogel daily disposable lens underneath the scleral lens for improved comfort and to prevent epithelial disruption. In most cases, corneal touch can be avoided by incorporating different diameters or altering specific quadrants of the lens if needed.
When fitting more complex cases, consider using EyePrintPro, an optically clear scleral cover shell that is created by taking a three-dimensional impression of the unique irregularities of an individual’s eye. A scleral lens with the exact impression of the ocular surface and individualized front surface optics may help optimize vision and comfort for each patient while preventing further issues.
Minimizing Complications
Similar to other contact lenses, scleral lenses, if fit or worn improperly, can lead to conjunctival hyperemia and corneal or conjunctival staining. Conjunctival hyperemia can be an indication of a tight periphery or a toxic reaction to preservatives. In cases like this, refit the patient with a flatter periphery and confirm they are using nonpreserved saline (and disposing after a single use) and the recommended cleaning solutions. Advise patients to bring their supplies to the office, including plungers or other insertion tools, to review product compliance.
Conjunctival hyperemia can result from conjunctival vascular impingement, which occurs when the lens edge pinches the conjunctival tissue; as such, a flatter scleral landing zone may minimize this issue.6 Asymmetrical back surface lens designs with quadrant specific technology or toric landing zones can also address localized conjunctival impingement. Conversely, scleral lens compression—excessive bearing of the scleral lens on the conjunctiva—can result in conjunctival blanching.6 However, rebound hyperemia may occur at the location of the compression following lens removal.6 Scleral lens compression can result from midperipheral curves that are too steep.7 Therefore, flattening the midperipheral curves should resolve this problem.
If localized conjunctival and/or corneal staining is present, consider not only the possibility of an improper lens fit but also mechanical involvement due to handling issues as the cause of the staining. If handling issues are a concern, re-educate the patient on proper removal to prevent mechanical injury. Any diffuse staining observed is likely due to a toxic reaction to the preservatives. Move the patient to 0.9% sodium chloride inhalation solution or the recently FDA-approved LacriPure (Menicon) in a single-use vial, and advise the patient to dispose of the vial after a single use.
Scleral lens-induced conjunctival prolapse appears to be a relatively benign complication. It occurs when conjunctival tissue is pulled under the lens and onto the peripheral cornea or limbus in areas of significant lens clearance, especially inferiorly.8 Significant inferior lens clearance is most frequently present when the scleral lens positions inferiorly or in cases of inferior corneal depression, most often seen in keratoconus and pellucid marginal degeneration. Though transient conjunctival prolapse appears to be benign, the long-term effects of chronic conjunctival prolapse are unknown. However, the conjunctival tissue may impede fluid exchange beneath the lens.9 Additionally, the prolapsed conjunctiva can eventually adhere to the corneal surface such that it cannot be easily manipulated. Therefore, we recommend addressing this issue early on. Making use of non-rotationally symmetrical designs may help minimize areas of excessive limbal or edge clearance. If conjunctival prolapse is present, anesthetize the eye and use a cotton tip applicator or forceps to pull back the conjunctiva from the cornea.8
Though little is known about the long-term effects of scleral lenses on corneal physiology, recent studies suggest the presence of an excessively deep fluid reservoir along with thick lenses may lead to hypoxia-induced corneal swelling.10,11 To avoid this, researchers have proposed to incorporate lens materials of the highest Dk available and design the lens with a maximum central thickness of 250μm and an apical clearance of 200μm.10,11 Nonetheless, to our knowledge, larger diameter gas permeable lenses with apical clearances greater than 200μm have worked successfully in patients. We believe as we learn more about scleral lens hypoxia-induced corneal edema with future research, we will be better able to design scleral lenses with less concern regarding this potential complication.
As with any lenses, challenges may arise with scleral lens wear. These simple tips can help address patient concerns and ensure success with these designs. Above all, however, communicate the how and why of scleral lens selection, use and complications to your patient. Doing so can earn your practice a patient for life.
Dr. Mickles is an associate professor at Nova Southeastern University College of Optometry in Fort Lauderdale, Fla. She lectures and publishes on a variety of topics with special interest in contact lenses and dry eye disease. She is a fellow of the American Academy of Optometry.
Dr. Barnett is a principal optometrist at the UC Davis Eye Center in Sacramento, Calif. She lectures and publishes extensively on topics including dry eye, anterior segment disease and contact lenses. Dr. Barnett serves on the board of Women of Vision, the Gas Permeable Lens Institute and the Ocular Surface Society of Optometry.She is president of the Scleral Lens Education Society.
1. Nichols J. Contact lenses 2015 annual report. Contact Lens Spectrum. Jan 2016;31:18-23.
2. Visser ES, Visser R, Van Lier HFF, Otten HM. Modern scleral lenses part II: patient satisfaction. Eye & Contact Lens. 2007;33(1):21-25.
3. Walker M, Morrison S, Caroline P, et al. Laboratory analysis of scleral lens tear reservoir clouding. Poster presented at the 2014 Global Specialty Lens Symposium, Las Vegas, Jan 2014.
4. Liu J, Sheha H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allerg Clin Immunol. 2010;10:505-510.
5. Bennett, ES, Scheid, T, Morgan, BW. “Gas-Permeable Lens Problem Solving.” Clinical Manual of Contact lenses. Philadelphia: Lippincott Williams & Wilkins; 2014:203-228.
6. van der Worp E. A Guide to Scleral Lens Fitting, Version 2.0 [monograph online]. Forest Grove, OR: Pacific University; 2015.
7. Denaeyer, GW. The scleral vault. Contact Lens Spectrum. Mar 2015;30:40
8. Caroline, PJ, Andre, MP. A potential effect of conjunctival prolapse. Contact Lens Spectrum. Sep 2013;28(9):56.
9. Severinsky B, Behrman S, Frucht-Pery J, Solomon A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty: Long term outcomes. Cont Lens Anterior Eye. 2014 Jun;37:196-202.
10. Jaynes JM, Edrington TB, Weissman BA. Predicting scleral GP lens entrapped tear layer oxygen tensions. Cont Lens Anterior Eye. 2015 Feb;38:44-47.
11. Michaud L, van der Worp E, Brazeau D, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Cont. Lens Anterior Eye. 2012 Dec;35(6):266-71.


