 |
Consider the following situation: One of your established patients who has successfully worn contact lenses for years presents with bilateral contact lens intolerance and red eyes. Has the patient gone rogue and started using an inferior cleaning regimen or suddenly started overwearing the lenses? Perhaps, but an adverse ocular effect from a systemic drug should also be on your radar, especially if they recently started a new medication.
The eye is prone to drug-related adverse effects due, in part, to its extensive blood supply and relatively small mass.1 Elderly patients are especially susceptible because of the increased likelihood of using multiple systemic medications for chronic conditions and impaired ability to metabolize and excrete medications.1
We are very familiar with the potential of certain oral medications to cause ocular sequelae such as hydroxychloroquine, prednisone, topiramate, tamoxifen and amiodarone. But could lesser-known medications cause ocular side effects? How would you know?
This question can be overwhelming, as the United States Food and Drug Administration (FDA) Center for Drug Evaluation and Research approves hundreds of new medications each year. While most are existing products that have new approvals, from 2009 to 2016, the FDA approved 251 new molecular entities.2 In 2017 alone, 26 new drugs and biological products have already been approved.2 Additionally, these approvals do not include the vast array of herbals and nutritional supplements that do not go through the FDA approval process.
As such, there is much to learn about new medications and supplements and their effects on the eye.
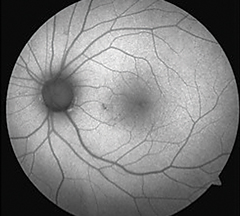 |
| Hydroxychloroquine is a well-known medication that can cause toxicity, shown here as an area of hypoautofluorescence nasal paracentral to the macula. But what other systemic medications and supplements should clinicians be on the lookout for? Photo: Beth Norris, OD, and Sara Henney, OD |
Available Resources
It is often challenging to determine if a systemic medication is causing an ocular condition. One useful resource is the book Drug-Induced Ocular Side Effects, which was written to help practitioners determine if a visual condition is related to a medication, an herbal supplement or another chemical.3 Ocular side effects are gathered from reports obtained by the FDA, MedWatch, the National Registry of Drug-Induced Ocular Side Effects (NRDIOSE) and the World Health Organization (WHO). The WHO Causality Assessment Guide of Suspected Adverse Reactions is then used to classify the reported adverse drug-related events as certain, probable, possible, unlikely or unclassified.3
As the number of systemic medications is ever-increasing, it is difficult for literature to track every potential adverse event. When practitioners observe an ocular adverse event from a systemic medication that has not been previously documented, they should report it at www.eyedrugregistry.com or www.fda.gov/safety/medwatch. Another beneficial resource is the NRDIOSE, which provides practitioners with data on any drug that has significant ocular side effects, as well as updated literature on particular drugs.4
Supplements
Practitioners should ask patients about herbal and nutritional supplement use, as that could be another cause of ocular issues. Chamomile and psyllium have shown to cause nonspecific conjunctivitis, while niacin can cause dry eyes.3,5 Jimson weed (Datura stramonium), an annual herb used for a wide variety of conditions including asthma, analgesia and motion sickness, contains high concentrations of atropine and scopolamine, making it a potential cause of pupil dilation.5,6
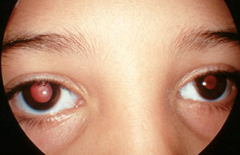 |
| Handling plants such as Jimson weed, which contains atropine and scopolamine, is the likely cause of this patient’s dilated tonic pupil OD. Photo: Joseph W. Sowka, OD, and Alan G. Kabat, OD |
A Diagnosis of Exclusion
Even if a patient is taking a systemic medication that has been linked to ocular adverse effects, other pathologies should still be ruled out first. When trying to determine whether an ocular side effect is linked to a systemic medication, it is essential to obtain a thorough medication history either from the patient or an electronic health record (if linked to the patient’s medication history).
Take bisphosphonate calcium regulating agents, for example. They are most often used in the management of osteoporosis and include many well-known medications such as Fosamax (alendronate, Merck), Actonel (risedronate sodium, Proctor & Gamble Pharmaceuticals), Boniva (ibandronate, Roche Laboratories) and Zometa (zolendronic acid, Novartis). Ocular adverse effects of these medications include blurred vision, transient conjunctivitis, uveitis and scleritis. This does not imply that every patient will get these side effects, but if a patient does present with one of these ocular manifestations within a few days of beginning therapy with a bisphosphonate, it may be attributed to the medication.
It is imperative for practitioners to be aware of which common systemic drugs can cause adverse ocular effects. They must also maintain a high level of suspicion when looking at a patient’s medication history. It is important to realize that even in the absence of officially-reported ocular adverse events, systemic medications may be affecting the ocular health of patients. Because many of the medications linked to ocular side effects are prescribed by other providers and may not be able to be discontinued, practitioners should communicate both the findings and subsequent management plans to the patient’s primary care practitioner or specialists. Most of these adverse events are infrequent, but they do occur and we need to be on the alert.
1. Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications. Drugs. 2007;67(1):75-93.2. Drugs@FDA: FDA approved drug products. FDA.gov. www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=report.page. Accessed July 30, 2017.
2. New drugs at FDA: CDER’s new molecular entities and new therapeutic biological products. FDA.gov. www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/default.htm. Accessed July 30, 2017.
3. Fraunfelder FT, Fraunfelder FW, Chambers WA. Drug-induced ocular side effects. 7th ed. Philadelphia: Elsevier Saunders;2015.
4. National Registry of Drug-Induced Ocular Side Effects. www.eyedrugregistry.com. Accessed July 29, 2017.
5. Wilkinson JT, Fraunfelder FW. Use of herbal medicines and nutritional supplements in ocular disorders: an evidence-based review. Drugs. 2011;1(18):2421-34.
6. Soni P, Siddiqui AA, Dwivedi J, Soni V. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: an overview. Asian Pac J Trop Biomed. 2012;2(12):1002-8.


