 |
In 2008, the National Institutes of Health launched the Human Microbiome Project with the purpose of identifying the organisms that live both on and inside the human species. The ocular surface was of particular interest, and the project estimated that over 200 species of bacteria colonize the human conjunctiva. Among the most typical species were Staphylococcus, Streptococcus and Corynebacterium—all gram-positive. Pseudomonas and Neisseria were those identified among the gram-negative organisms.1,2
The purpose of this complex community of organisms is somewhat difficult to discern. Given that we mostly live symbiotically with these microbes, it’s possible they are present to prevent more severe organisms from attacking. As an example, Clostridium difficile is present in the intestines but is held in check by other normal flora until something, such as overuse of antibiotics, erases the natural population, providing C. diff. a window of opportunity.
The ocular surface has similar complexities. The ocular surface’s biofilm lifecycle varies from days to weeks, depending on the organisms present and is associated, in most instances, with mucins that have evolved with us over millennia.
Over the last decade, we have seen significant erosion of the efficacy of commonly used agents, especially in the gram-positive arena.
The ARMOR study analyzed 3,237 bacterial isolates at 22 sites from 2009-2013. It looked at isolates common to the ocular surface (Streptococcus pneumonia, Staphylococcus aureus, coagulase-negative Staphylococci, Pseudomonas aeruginosa and Haemophilus influenza), as well as commonly used antibiotics, including fluoroquinolones, aminoglycosides, macrolides, cephalosporins and penicillins.3
Corneal Disease
Beginning almost two decades ago with earlier generations (ofloxacin and ciprofloxacin), fluoroquinolones evolved into the go to drugs for the treatment of corneal disease, including microbial keratitis. Typical treatment protocols varied from QID to Q 1 hour, depending on disease severity. As use increased, the efficacy against gram-positive isolates (specifically Staph.) began to erode, and the next generation agents, gatifloxacin and moxifloxacin, replaced their precursors and were considered incapable of developing resistance due to the necessity of a dual, simultaneous mutation.5 Unfortunately, this proved untrue and today the resistance to both generations by methicillin-resistant Staphylococcus aureus (MRSA) is extremely high; some studies indicate less than 20% efficacy against these organisms.6
Commonly, clinicians initiate microbial keratitis treatment with a fluoroquinolone on a Q 1 hour to Q 3 hour basis, depending on the severity of presentation. But data on methicillin-resistant isolates demonstrates the need for vigilance after the first 24 to 48 hours of therapy in patients who have not demonstrated clinical stabilization or improvement.3 In those cases, the best alternative is fortified vancomycin (30mg/ml) in addition to the fluoroquinolone on a Q 1 hour or Q 1/2 hour basis. According to ARMOR, fluoroquinolones have a low level of sensitivity to gram-positive MRSA while maintaining good sensitivity to other gram-positive isolates as well as the more typical gram-negative isolates such as Pseudomonas. So, if treatment fails to improve the patient’s condition, the most likely cause is a non-sensitive gram-positive organism. Additionally, the study demonstrated excellent MRSA coverage for vancomycin.
Another concern is that the best agents are not available on patients’ formulary or they cost too much. Thus, it’s useful to know which other agents can be used. Data show that Polytrim (trimethoprim/polymixin B sulfate, Allergan) maintains good sensitivity to MRSA and that earlier generation fluoroquinolones are reasonably similar to the next generation for initial ulcer treatment when newer generation agents are not available at effectively the same dosage patterns.3,4,6
Conjunctivitis and Blepharitis
When treating common conjunctivitis, the ARMOR study indicates that typical therapy patterns have not changed materially other than the notable decline in the sensitivity of tobramycin and macrolides.6 While these agents may show success in some patients, if the patient’s condition does not improve, it is most likely the consequence of increased resistance, making selection of an alternative agent, such as Polytrim, appropriate. Additionally, clinicians can consider using Neosporin Ophthalmic Solution (neomycin and polymyxin B sulfates, and gramicidin, Pfizer) given the relative increase in sensitivity generated by its absence in the ophthalmic market.
In the treatment of blepharitis, several studies show little improved efficacy in the use of antibiotics versus lid hygiene in long-term management of these conditions.7 Given the ARMOR data on the poor performance of tobramycin, look for other alternatives. Numerous new topical regimens for cleaning the lids and multiple versions of lid scrubs, allow practitioners to avoid antibiotic use altogether.
If I must use an antibiotic for lid-related disease, I select an ointment for night time use and combine it with aggressive lid hygiene. Agents such as Bacitracin (Pfizer), Polymyxin (Sagent Pharmaceuticals) or their combination have excellent coverage against typical isolates and have the advantage of being non-preserved. Several studies have looked at the treatment of ocular surface disease related to posterior lid disease for recurrent chalazia that involve the long-term use of re-esterified omega 3 fatty acids at approximately 3,000mg per day. This avoids the need for doxycycline, which has the potential for significant side effects as well the development of resistance.8
Preseptal Disease
With preseptal cellulitis, the organisms typically involved are similar to those seen in microbial keratitis rather than Pseudomonas. Since Staphylococcus and some Streptococcus primarily populate lids, selection of an appropriate agent should be based on the patient’s allergy history and the known performance of the antibiotic against Staph.
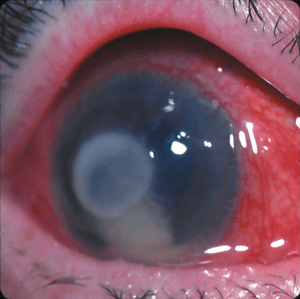 |
| Fourth-generation fluoroquinolones may be the most effective option against MRSA, which caused this patient’s corneal ulcer. |
In the penicillin class, drugs such as ampicillin and dicloxacillin at 1,000mg to 1,500mg per day are typically effective. Many clinicians prefer Augmentin (GlaxoSmithKline), a combination of ampicillin and clavulanate, which is a beta lactamase inhibitor that increases efficacy of therapy (875mg BID). As for the cephalosporin class, first generation agents have greater efficacy against gram-positive organisms than 2nd or 3rd generation agents. Keflex (Advancis Pharmaceutical) and Duricef (Bristol-Myers Squibb) are both effective and cost efficient for the majority of patients. The newer classes of drugs such as macrolides (clarithromycin) and fluoroquinolones have shown little improvement in outcomes but do demonstrate increased side effect profiles at a higher cost.9
In the United States, pathogens resistant to antibiotics cost us approximately $20 billion a year and add eight million additional hospital days. A 2014 WHO update shows continued erosion of drug efficacy in all key categories worldwide with continued loss of efficacy in the FQ and cephalosporin classes even in treatment of last resort settings.10 As eye care practitioners, it is critical we understand the impact of the market on our decisions and our patients. Antibiotic resistance is one of the biggest health issues, and we all must work toward changing the course of its impact on our patients and ourselves.
1. Abelson M, McLaughlin J. Of biomes, biofilm and the ocular Surface. Review of Ophthalmology. 2012;19(9):52-4.
2. Wilcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013 Dec;117:99-105.
3. Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic Resistance Among Ocular Pathogens in the United States: Five-Year Results From the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015 Dec;133(12):1445-54.
4. Asbell PA. ARVO/Healio.com. Opthalmology. May 8, 2015.
5. Scoper SV. Review of the third and fourth generation fluoroquinolones in ophthalmology: in vitro and in vivo efficacy. Adv Ther. 2008 Oct;25(10):979-94.
6. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145(6):951-8.
7. Lindley E, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012 May 16;(5):CD005556.
8. Epitropoulous AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016 Sep;35(9):1185-91.
9. Piccirillo F, Mager DE, Frisse ME, et al. Impact of first line vs second line antibiotics for the treatment of acute uncomplicated sinusitis. JAMA. 2001 Oct 17;286(15):1849-56.
10. The World Health Organization. WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. News Release. April 30, 2014. Available at www.who.int/mediacentre/news/releases/2014/amr-report/en/. Accessed August 22, 2016.


