Our understanding of the ocular surface has increased dramatically over the past few years, and much of the research has highlighted the role the eyelids and meibomian glands play in supporting a stable tear layer. At the same time, renewed interest in the causative factors for contact lens discomfort and dropout have fueled innovation in contact lens material formulation, tear chemistry and environmental factors impacting lens wear.
Disagreement abounds regarding the role meibomian gland dysfunction (MGD) and function play in contact lens wear. Contact lenses’ impact on the meibomian glands also remains the subject of ongoing debate. This article reviews the literature and explores current thinking regarding contact lenses and meibomian gland function, while examining the broader issues of tear film/contact lens interaction.
Understanding the Tears
While tears serve numerous well-recognized purposes, their primary functions are refractive and protective. This sometimes causes confusion among patients who assume tears exist only to keep the eyes wet and believe their absence leads to dry eye and discomfort. This can be especially confusing for contact lens wearers who mistake contact lens-related discomfort for ocular dryness.1 Similarly, eye care practitioners are also often confused regarding the cause of dry eye, which is now increasingly recognized as more of a misnomer than a descriptive diagnosis.
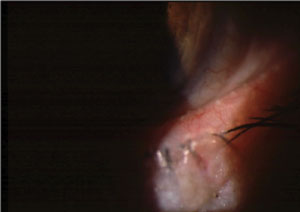 |
| MGD with telangiectatic vessels and inspissated meibomian glands. |
Reflex tears serve the critical purpose of flushing irritants and foreign bodies from the ocular surface before they cause significant damage to the corneal surface. This reduces the potential for abrasion and infection with subsequent loss of vision and function. In contrast, basal tears arising from the accessory glands of Krause and Wolfring, with possible contribution from the lacrimal glands, are structural in nature—an often overlooked property. The tear foundation transforms the naturally hydrophobic surface into a hydrophilic entity, allowing the tears to remain in place, defying gravity and other extrinsic and intrinsic forces. The majority of tears consist of a viscoelastic gel made up of mucins and other components. This structural layer offers protection and smoothing, and likely changes its behavior during different phases of the blink.
The outer layer is derived from the meibomian glands, which express a complex lipid with each blink; however, the function and clinical importance of meibum is not broadly understood. Hydrophobic bonding causes lipid to attract lipid. When sufficient lipid is present to cover the interpalpebral globe, a coherent layer stretches over the outer surface of the eye. The tear lipid layer provides significant structure to the tear film.
The interface between this outer, nonpolar lipid layer and the rest of the tears occurs through the interfacial action of phospholipids, which bond polar and nonpolar components, effectively anchoring the outer lipid layer to the mostly aqueous sub-layer below.
MGD
Dry eye has historically been viewed as the result of an aqueous tear deficiency.2 Normal meibomian gland function has increasingly been recognized as an essential element in the maintenance of ocular surface health and function. Contemporary literature confirms that MGD is a primary or major contributory factor in nearly 90% of patients complaining of dry eye—which is borne out in clinical practice.3 More recently, researchers questioned whether dry is more frequently an erroneous description of a disorder driven by abnormal meibomian gland function.4
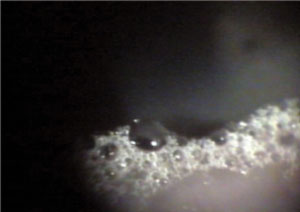 |
| Frothing due to saponification. |
MGD is defined as “a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. This may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease.”5
While the exact causes of MGD remain unclear and are likely multifactorial, healthy meibomian gland function is better understood. Driven by the force of the blink, normal gland expression produces a clear, free flowing, complex oil. Exiting through orifices on the lid margin, meibum intermixes with the tears, resulting in an effective evaporative barrier, decreased surface tension and friction and increased tear stability.
There are a greater number and length of glands in the upper eyelid, likely producing additional meibum compared with the lower lid. The glands are buried deep within the lids and protected by the tarsal plate, subcutaneous fat and overlaid by a robust vascular net. Meibum production occurs in the acini, the grape-like clusters present along the length of the glands and remains relatively constant. However, the amount of meibum delivered by each gland varies over time and tends to decrease with age.
Structural changes with MGD include atrophy, truncation, gland loss (which can be complete, sectoral or segmental) and convolution. Dilation of the distal duct, sometimes referred to as tooth sign, reflects obstruction and is likely caused by back-pressure.
Researchers postulate alterations in normal blink patterns and shifts in dietary intake of essential fatty acids are causal factors in MGD.6,7 Blink frequency and rate of blink completion is reduced during tasks requiring concentration, reading text and especially during computer use.8-10 Likewise, shifts in dietary intake over recent decades have resulted in an imbalance in omega-3 vs. omega-6 free fatty acids. Reduced levels of omega-3 fatty acids have been implicated as factors in the pathophysiology of MGD.
Contact Lens Wear
Researchers first described the association of MGD with contact lens intolerance in 1980.11 They reported obstructive meibomian gland disease due to blockage of the glands by desquamated epithelial cells as the cause of dryness and discomfort in intolerant contact lens wearers. Other studies report that a decrease in the number of functional meibomian glands in contact lens wearers worsens with duration of contact lens wear.12
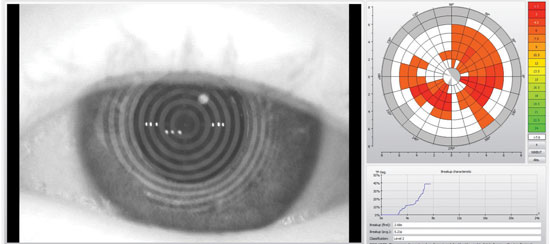 |
| Tear film scans provide non-invasive assessment of tear film break-up time. |
The association of contact lens wear with MGD still remains somewhat controversial—perhaps because of the complexity and multifactorial nature of MGD and contact lens interaction with the ocular environment. While several studies failed to demonstrate a link between MGD and contact lens wear, a recent report offers compelling evidence for the role contact lenses play in predisposing patients to meibomian gland and related lid abnormalities.13-16
Contact lenses can lead to MGD in a variety of ways. Blink rates and meibomian gland function may be affected by lens wear, intense visual tasks and concentration. With rigid lenses, blinking may decrease, resulting in lower amounts of meibomian gland secretions. In soft lens wearers, studies associate decreased tear stability and adverse environmental conditions with increased blinking, which may be an adaptive mechanism resulting in increased meibomian gland lipid production.17,18
Meibomian gland loss is strongly correlated with decreased lipid layer thickness and tear instability.19 Although meibomian glands are mechanically compressed and meibum expressed as a function of the blink, higher-order control is likely active in regulating tear lipid and aqueous levels. Decreased tear lipid levels result in tear instability and is likely to reactively prompt increased compensatory meibum and tear secretion in healthy patients.20,21
Typical contact lenses are significantly thicker than the normal tear film. This disparity causes the tears to essentially stretch to accommodate the relatively massive thickness of the contact lens, resulting in thinning of the pre- and post-lens tear film and destabilization of the tear structure. This can lead to a cycle of increasing tear instability and compensatory meibum overproduction, eventually taxing gland capability—especially in the presence of MGD or frank gland loss. It is possible, if not likely, that chronically overproducing meibomian glands can burn out, leading to damage and overall gland down-regulation and loss.
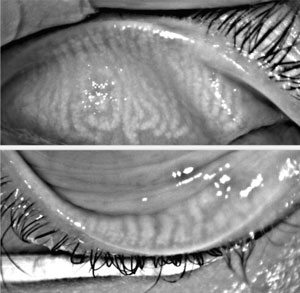 |
| Meibography shows the tooth sign on the distal upper and lower lid glands. |
Uptake and sequestration of lipids by hydrophobic moieties in contact lens materials can exacerbate the situation. Silicone hydrogel materials, especially early generation lenses, are known for their propensity to bind lipids onto their surfaces.22 Further, some modern contact lens materials are reportedly formulated to integrate lipids within their structure. While this may improve surface compatibility, it can also result in compensatory up-regulation of lipid production and an eventual adverse impact on meibomian glands.
Diagnosis and Management
Regardless of whether contact lens wear causes MGD or MGD causes contact lens discomfort and intolerance, comorbidity between the two is highly likely, if not certain. Since the overwhelming majority of dry eye is directly caused by or closely associated with MGD, contact lens wearers experiencing dryness and discomfort should always be evaluated for MGD. Prospective contact lens wearers who have MGD should be treated before initiating contact lens wear to help ensure success. Before attributing patient complaints to problems with their contact lenses or solutions, a thorough workup is indicated, including assessment of meibomian gland function and structural integrity.
An experienced clinician can evaluate meibomian gland function with diagnostic expression using either fingertip pressure applied to the lids or the Korb Meibomian Gland Evaluator (TearScience). Interferometry can assess meibomian gland lipid production as well as track changes during treatment. Because of the sensitivity of interferometry, clinicians must be careful when removing contact lenses before testing to avoid interference with lipid layer thickness readings.
Although transillumination of the lid margin can be helpful in evaluating gland structure, meibography is superior at documenting MGD-related gland loss and structural changes. The high-quality images generated by meibography are also a useful educational tool for patients in discussing the disease.
Tear break-up time is an extremely useful measure of tear stability and lipid layer integrity. Noninvasive measurements can be performed with lenses in place, which can reveal important information.
Both traditional and contemporary MGD management approaches may be useful for symptomatic contact lens patients who have MGD. Lid scrubs can be beneficial in managing symptomatic contact lens wearers.23 More recently, several studies (including an as yet unpublished multicenter study) have investigated the use of LipiFlow (TearScience) thermal pulsation therapy in contact lens wearers with MGD. Researchers saw significant improvement in MGD scores and patient symptoms after a single treatment.24 Another device that applies heat to reportedly improve gland expression is the MiBoFlo Thermoflo.
Pure hypochlorous acid applied to the lids in products such as Avenova (Novabay) may be beneficial by triggering antimicrobial activity on lid flora overpopulation and inhibition of associated lipolysis in the tear film.25 Omega-3 supplements have also proven helpful in managing MGD.26 A recently published study investigating the role of oral re-esterified omega-3 nutritional supplementation on dry eyes showed significant treatment benefits.27
Beyond identifying and managing MGD, clinicians must be aware of the role different contact lens materials play in exacerbating lens-related evaporative dry eye. We should prescribe materials that adsorb significant amounts of lipid cautiously. Solutions and lens materials designed to increase surface wettability may help reduce the potential adverse impact of lens wear on MGD.
BLINK EXERCISES CLOSE - PAUSE - PAUSE - OPEN
1. Hold your fingers at the corners of your eyes and blink. If you feel anything, you are using your defense muscles that run along the side of your head. Your blinking muscles are above your eyelids. 2. Read the blinking sequence. It is very important to do the pause step to make complete contact between the upper and lower lids (partial blinking is very common in people with dry eyes). When you are doing it correctly, you should feel no movement under your fingers. 3. Blinking is very task-dependent. For example, if you spend a lot of time on the computer, you are probably blinking much less frequently and might want to post a copy of the blinking exercises nearby as a friendly reminder. (Other pastimes that decrease blink rate are driving, reading, watching TV, working at your desk, or any concentrated visual task, etc.) 4. Lastly, if you having difficulty consciously incorporating the blinking exercise into your schedule (i.e. 5X/hour) you might want to think about something you do often in your daily routine, such as answering phone calls, sending emails, drinking sips of water, getting dressed, etc. If you can condition yourself to make a full blink and give a little squeeze every time you perform this action it helps to make complete blinking a habit. | |
| TearScience’s Donald Korb Blink Training app offers directions to help patients with MGD learn proper blink technique to mitigate symptoms. |
Clinician Takeaway
A steady increase of patients presenting with dry eye threatens to complicate contact lens management. While changes in visual demands and diet likely play significant roles, awareness of MGD and the resulting tear dysfunction helps the clinician troubleshoot and address patients’ discomfort and dryness. With ever-increasing patient expectations and an aging population, understanding risk factors and appropriate MGD management will become increasingly vital tools in the armamentarium of the successful contact lens fitter.
Dr. Epstein is co-founder of Phoenix Eye Care, where he heads the Ocular Surface Disease Center and serves as the Center’s director of clinical research.
1. Kadence International. Exploring Comfort and Vision Survey. 2012.2. Murube J. History of the Dry Eye. In: The Dry Eye: A Comprehensive Guide. Lemp MA, Marquardt R, eds. Heidelberg, Germany: Springer; 1992:3-34.
3. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8.
4. Korb DR, Blackie CA. “Dry eye” is the wrong diagnosis for millions. Optom Vis Sci. 2015;92(9):e350-4.
5. The International Workshop on Meibomian Gland Dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):1917–2085.
6. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15(4):340-6.
7. Schafer J, Steffen R, Reindel W, Chinn J. Evaluation of surface water characteristics of novel daily disposable contact lens materials, using refractive index shifts after wear. Clin Ophthalmol. 2015;9:1973-9.
8. Argilés M, Cardona G, Pérez-Cabré E, Rodríguez M. Blink rate and incomplete blinks in six different controlled hard-copy and electronic reading conditions. Invest Ophthalmol Vis Sci. 2015;56(11):6679-85.
9. Chu CA, Rosenfield M, Portello JK. Blink patterns: reading from a computer screen versus hard copy. Optom Vis Sci. 2014;91(3):297-302.
10. Patel S, Henderson R, Bradley L, et al. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci. 1991;68:11:888-92.
11. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51(3):243-51.
12. Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116(3):379-84.
13. Ong BL. Relation between contact lens wear and meibomian gland dysfunction. Optom Vis Sci. 1996;73(3):208-10.
14. Marren SE. Contact lens wear, use of eye cosmetics, and meibomian gland dysfunction. Optom Vis Sci. 1994;71(1):60-2.
15. Pucker AD, Jones-Jordan LA, Li W, et al. Associations with meibomian gland atrophy in daily contact lens wearers. Optom Vis Sci. 2015;92(9):e206-13.
16. Machalińska A, Zakrzewska A, Adamek B, et al. Comparison of morphological and functional meibomian gland characteristics between daily contact lens wearers and nonwearers. Cornea. 2015;34(9):1098-104.
17. Kojima T, Matsumoto Y, Ibrahim OM, et al. Effect of controlled adverse chamber environment exposure on tear functions in silicon hydrogel and hydrogel soft contact lens wearers. Invest Ophthalmol Vis Sci. 2011;52(12):8811-7.
18. Jansen ME, Begley CG, Himebaugh NH, Port NL. Effect of contact lens wear and a near task on tear film break-up. Optom Vis Sci. 2010;87(5):350-7.
19. Eom Y, Lee JS, Kang SY, et al. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155(6):1104-10.
20. Arita R, Morishige N, Koh S, et al. Increased tear fluid production as a compensatory response to meibomian gland loss: a multicenter cross-sectional study. Ophthalmology. 2015;122(5):925-33.
21. Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300-6.
22. Nichols JJ. Deposition on silicone hydrogel lenses. Eye Contact Lens. 2013;39(1):20-3.
23. Paugh JR, Knapp LL, Martinson JR, et al. Meibomian therapy in problematic contact lens wear. Optom Vis Sci. 1990;67(11):803-6.
24. American Academy of Optometry. A single lipiflow treatment increases soft contact lens wearing time and reduces lid wiper epitheliopathy and dry eye symptoms. Available at www.aaopt.org/single-lipiflow-treatment-increases-soft-contact-lens-wearing-time-and-reduces-lid-wiper. Accessed September 15, 2016.
25. Epstein A, Pang L, Noorbakhsh C, et al. Comparison of bacterial lipase activity in the presence of eye lid cleansers. Available at http://novabay.com/wp-content/uploads/2016/07/Epstein-ARVO-2015-Comparison-of-Bacterial-Lipase-Activity-in-the-Presence-of-Eye-Lid-Cleansers.pdf. Accessed September 15, 2016.
26. Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:336-56.
27. Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185-91.