Dry eye disease (DED) is a massive but often under-appreciated problem. Data suggests as many as 35% of Americans have some degree of DED.1,2 While there is a surfeit of information on DED treatment, diagnosis and observation seem to be the more challenging aspects.
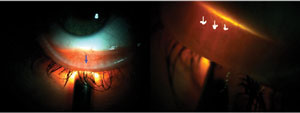 |
| Fig. 1. Transillumination of lower lid, showing meibomian gland structure with blue and white arrows. |
The ocular tear film is often observed, but rarely qualified. This qualification is one of the most challenging parts of managing DED. The ocular tear film provides lubrication and visual clarity and is also the first barrier of protection for our eyes. Any vehicle is markedly limited without a windshield, but if the windshield it has is covered in dirt or mud, it is useless.
Knowing the anatomical characteristics of the tear film builds the framework for a better understanding of ocular surface disease.
Tear Film Anatomy: A Refresher
All clinicians know that the tear film is comprised of three dynamic layers—mucin, aqueous and lipid—but some of its specifics may elude us in routine practice. The mucin layer is 0.02µm to 0.05µm thick and provides an anchor for tear film foundation, while the aqueous layer resides between both mucin and lipid layers and is the thickest layer, at 0.7µm. The most superficial is the lipid layer, which helps protect the tear film from decomposition.3
Tears are produced from the lacrimal gland under the influence of the parasympathetic and sympathetic nervous system.4 Through dynamic tear film interferometry we know tear fluid is spread over the ocular surface from temporal to the nasal segments of the eye.5,6 The distribution of tears is dependent on lid blink, and facilitation of drainage is through the lacrimal puncta.5,7 The tear film takes one of three outputs: evaporation, drainage or absorption. Evaporation is inevitable, but accelerated by poor lipid tear layer.8 Drainage through the nasolacrimal system can be impeded by anatomical morphology such as scarring or cautery. Absorption is suggested through corneal permeability in the absence of compromised corneal function and is reported as minimal, roughly 15% absorption.9,10
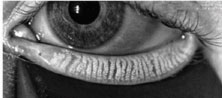 |
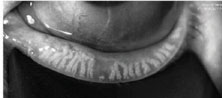 |
| Fig. 2. Meibography of normal gland structure (top) and gland truncation or scarring (bottom). |
Collectively, the tear film supplies the ocular surface with many nutrients and wound healing properties such as fibronectin, vitamins and growth factors.11 These elements support and modulate proliferation, migration and differentiation of the conjunctival and corneal epithelium.
Aqueous-Deficient vs. Evaporative DED
The Dry Eye Workshop defined two modalities of DED: aqueous deficient and evaporative.12 Of these two subcategories, evaporative dry eye represents the majority of cases.13,14 Evaporative dry eye disease, which results from increased ocular surface exposure and meibomian gland dysfunction (MGD), is commonly associated with an inflammatory process.12
During inflammation, proteins are released into the tears, which serve to support opsonization (i.e., targeting) and phagocytosis of microbes by macrophages and lymphocytes. Ultimately, damage to the meibomian or sebaceous glands comes from androgen dysregulation, microbe invasion and excretory duct obstruction due to hyperkeratinization and increased viscosity of meibum.15,16 Tears serve a lubricating and mechanical clearance function, but also possess epitheliotropic and antimicrobial properties.17,18
The importance of identifying these underlying processes and how they contribute to tear film dysfunction cannot be overstated. Diagnostic tools have advanced to allow proper quality assessment of tear film dynamics, and they help to standardize a protocol for diagnosis and management.
Observing the Glands
Meibomian gland observation can be performed by meiboscopy, interferometry and meibography.
Meiboscopy is performed with basic transillumination of the lids (Figure 1).20 Although this method is quick, effective and available to all practitioners, newer techniques and tools provide better visualization of meibomian gland structure.
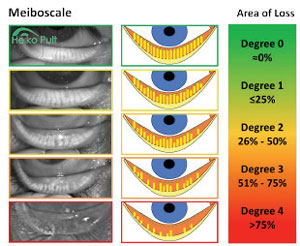 |
| Fig. 3. Five-grade meiboscale.14 © 2012, 2016 Dr. Heiko Pult — Optometry & Vision Research, Germany, www.heiko-pult-de, used with permission. |
Meibography allows for the evaluation of the number and morphology of the meibomian glands from their point of origin through to the ductal termination at the orifices (Figure 2). There are two principles in meibography: transillumination of the everted lid and direct illumination, or non-contact meibography. Slit-lamp microscopy (Topcon), portable noncontact meibography (Shenzhen LYD Technology), LipiScan (TearScience), LipiView II (TearScience) and Keratography 5M (Oculus) are all instruments designed to perform meibography.
Only gross imaging scales exist for grading at this time (Figure 3).21 Although rudimentary, they are effective in documenting baseline meibomian status by comparing the patient’s gland structure with the closest associated grading. Studies show methods of grading meibography images demonstrate good within-reader reliability and fair between-reader reliability.22 Researchers observed normal aging changes of meibomian glands such as acinar (glandular) density reduction and an evident decrease of the acinar diameter without significant modifications of the glandular orifice diameter.23 They also observed morphological changes in disease states; with MGD, acinar density was reduced but diameter was increased, and orifice diameter was dilated.23
Interferometry allows for micro observation of the tear film’s lipid layer. Basic optical theory of reflectance and thin film interferometry can indicate the hue and saturation seen as a function of the thickness of the transparent layer, causing the interference phenomena.24 Lipiview II (TearScience) is the only commercial instrument designed to provide real-time visualization of the lipid layer thickness, lid closure and blink rate with one scan. Research shows a thin lipid layer correlates with dry eye disease symptoms.25
Hyperosmolarity
Tear film hyperosmolarity is considered by some researchers to be the primary cause of discomfort, ocular surface damage and inflammation in dry eye.26 Osmolarity can be measured with TearLab’s handheld osmometer. A clinical cut-off of 312mOsm is suggested as the diagnostic level for aqueous-deficient and evaporative dry eye.27 Any decrease in meibum or aqueous secretion contributes to increases in osmolarity, thus complicating diagnostic specificity.
Researchers evaluated whether tear film osmolarity could be used as a reliable diagnostic tool for dry eye in patients with rheumatoid arthritis (RA). They found that 66% of patients with RA have osmolarity of greater than 316mOsm, serving as an indication for work-up of systemic inflammatory diseases.28
Matrix metalloproteinase-9 (MMP-9)—a nonspecific marker of inflammation—is a proteolytic enzyme that comes from stressed epithelial cells on the ocular surface.29 In healthy control individuals, MMP-9 is normally found at low levels; however, in those with DED, levels rise to more than 40ng/ml.29,30 MMP-9 can be measured by InflammaDry (Rapid Pathogen Screening, RPS). To perform this point-of-care test, swab the palpebral conjunctiva, and place the swab into the RPS cassette, where a buffer is applied to complete the test.
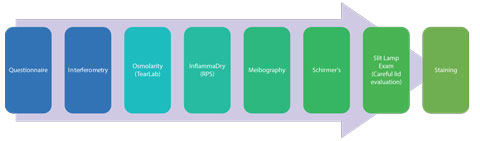 |
| Fig 4. Example of an ocular surface work-up protocol. |
Although eye care practitioners have long been able to identify dry eye patients by clinical observation of ocular surface disruption, osmolarity and MMP-9 testing—particularly when used in combination—increase the precision we can bring to our assessment and management plan.
Standardized Protocol
Diagnostic technologies still lack the ability to determine the ideal course of disease management—that remains a clinical responsibility. As providers, we seek certainty to provide the most targeted and cost effective care. Not all technologies provide equal value, but all increase our knowledge.
The best strategy with DED testing is to keep follow-up care consistent with a standardized protocol (Figure 4) that provides information about tear production, ocular surface grade and meibomian gland function.
Dr. Smith is an optometric physician with a special interest in dry eye management, therapeutic contact lenses and surgical comanagement. Currently, he practices at the Veterans Affairs outpatient clinic in Jacksonville, VA, where there is great demand for emergency eye care and management of chronic ocular disease. Dr. Smith has served as a board member and volunteer for many organizations, including: Volunteer Optometric Services to Humanity (VOSH), Jefferson County Community Participation Board and Equal Access Birmingham. He is a member of Florida Optometric Association and American Optometric Association and is a National Association of Veterans Affairs optometrist.
1. Improving screening, Diagnosis, And Treatment of Dry Eye Disease: Expert Recommendations From the 2014 Dry Eye Summit. National Eye Institute. Facts about dry eye. Availabe at www.nei.nih.gov/health/dryeye/dryeye. Accessed September 7, 2016.
2. The Epidemiology of Dry Eye Disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. 2007;5(2):93-107.
3. Creech J, Do L, Ratt I, Radke C. In vivo tear film thickness and implications for tear film stability. Curr Eye Res. 1998 Nov;(11):1058-66.
4. Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. The Ocular Surface. 2004;2:76-88.
5. Clinch TE, Benedetto DA, Felberg NT, Laibson PR. Schirmer’s test. A closer look. Arch Ophthalmol. 1983;101:1383-6.
6. Doane MG. An instrument for in vivo tear film interferometry. Optom Vis Sci. 1989;66:383-8.
7. Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17:565-96.
8. Lozato PA, Pisella PJ, Baudouin C. The lipid layer of the lacrimal tear film: physiology and pathology. J Fr Ophtalmol. 2001;24(6):643-58.
9. Joshi A, Maurice DM, Paugh JR. A new method for determining corneal epithelial barrier to fluorescein in humans. Invest Ophthalmol Vis Sci. 1996;37:1008-16.
10. Goebbels M, Spitznas M. Corneal epithelial permeability of dry eyes before and after treatment with artificial tears. Ophthalmology. 1992;99:873-8.
11. Geerling G, MacLennan S, Hartwig D. Autologous serum eyedrops for ocular surface disorders. Br J Ophthalmol. 2004;88(11):1467–74.
12. The definition and classification of dry eye disease: report of the Definition and classification Subcommitee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):75-92.
13. Shimazaki J, Sakata M, Tsubota D. Ocular surface changes and discomfort in patients with Meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266-70.
14. Lemp M, Crews L, Ron A, et al. Distribution of aqueous deficient and evaporative dry eye in a clinic-based patient population. Cornea. 2012 May;31(5):472-8.
15. Gilbard JP. The diagnosis and management of dry eyes. Otolargngol Clin N Am. 2005;38;5:871-85.
16. Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383-416.
17. Sullivan D, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41:3732-42.
18. Ng V, Cho P, Mak S, Lee A. Variability of tear protein levels in normal young adults: between-day variation. Graefes Arch Clin Exp Ophthalmol. 2000;238:892–9.
19. Liu S, Richards S, Lo K, et al. Changes in gene expression in human meibomian gland dysfunction. Inv Ophthmol Vis Sci. 2011;52:272-4.
20. Tapie R. Etude biomicroscopique des glandes de meibomius. Ann Oculistique. 1977;210:637–48.
21. Pult H, Riede-Pult B. Neues zur Meibographie. Die Kontaktlinse. 2011;6:24–5.
22. Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24:382–8.
23. Fasanella V, Agnifili L, Mastropasqua R, et al. In vivo laser scanning confocal microscopy of human meibomian glands in aging and ocular surface diseases. Biomed Res Int. 2016;7432131.
24. Doane M, Lee E. Tear film interferometry as a diagnostic tool for evaluating normal and dry-eye tear film. In: Lacrimal gland, tear film, and dry eye syndromes 2. 1998;438:297-303.
25. Tomlinson A, Khanai S. Assessment of tear film dynamics: quanification approach. 2005;3(2):81-95.
26. Blackie CA, Solomon JD, Scaffidi RC, et al. The relationship between dry eye symptoms and lipid layer thickness. Cornea. 2009;28(7):789-94.
27. Gilbard JP, Farris RL, Santamaria J 2nd. Osomolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:688-91.
28. Schargus M, Wolf F, Tony H, et al. Correlation between tear film osmolarity, dry eye disease, and rheumatoid arthritis. Cornea. 2014;33:1257-61.
29. Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of MMP-9 on the ocular surface increase in DTS. Invest Ophthalmol Vis Sci. 2009;50:3203-9.
30. Acera A, Rocha G, Vecino E, et al. Inflammatory markers in the tears of patients with OSD. Ophthalmic Res. 2008;40:315-21.


