We and our patients are fortunate to live in an age where we have a variety of contact lens options designed to improve vision and comfort, and promote ocular surface health. These specialty lenses are truly different and, as such, require special care.
Scleral Contact Lenses
Currently, scleral gas permeable (GP) contact lenses represent the fastest growing segment of the specialty contact lens industry.1 Already invaluable for treating patients with keratoconus and other corneal irregularities, scleral lenses are now also being worn by healthy patients who require simple refractive correction.
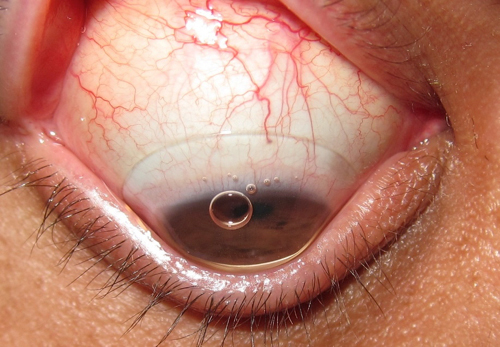 | |
| Fig. 1. Scleral contact lens with insertion bubble. |
Inherently larger than corneal GP lenses, sclerals are designed to vault the cornea and rest on the sclera. As such, they must be filled with solution prior to application to prevent air bubbles from forming underneath the lens (Figure 1), which can compromise comfort, vision and corneal health (i.e., compression of the epithelium and differential oxygen levels). In order to prevent this solution from spilling during application, patients should be instructed to keep their head down, parallel to the ground. In this position, the patient should open both eyelids wide (scleral lenses average about 16.0mm in diameter), gently place the lens on the conjunctiva and then close the eyelids.2
Note a scleral lens’s thickness (approximately 0.3mm), diameter and depth can affect its center of gravity, making it more difficult to balance the lens on one finger compared with a soft or corneal GP lens. As such, a number of methods may be utilized to assist in holding the lens for proper insertion:
• The “tripod method.” After forming a tripod with the thumb, index finger and middle finger, rest the lens in the center of the three digits for application.
• A large DMV or suction cup. As a recommendation, cut a small slice off the bottom or order the suction cup fenestrated so that it will be easier to remove from the lens following placement on the ocular surface.
• A #8 O-ring. Available from GP lens manufacturers or at many hardware stores. Before application, place the ring on the tip of the index finger and place the lens on top of the ring.3
• Ezi Scleral Lens Applicator (Q-Case). A ring-like device equipped with a bowl on which to balance the scleral lens during application.
• See Green Lens Inserter (Dalsey Adaptives). A device equipped with a permanent standing suction cup and green light to help focus the patient’s gaze during application (Figure 2). According to the manufacturer, the device is particularly suited for patients who struggle with manual dexterity, are monocular and cannot see the lens, or need to hold their eyelids.4
.jpg) | |
| Fig. 2. The Sea Green Lens Inserter with stand (Dalsey Adaptives). |
Filling the Lens
Scleral lenses provide minimal tear exchange, meaning the solution placed in the bowl of the lens prior to application remains in direct contact with the cornea during most of the lens-wearing day; thus, it is critical to use a nonpreserved solution to prevent preservatives from inducing allergic or hypersensitivity reactions.5,6
Scleral lenses are commonly filled with unit-dose sodium chloride 0.9% inhalation/irrigation solution, which can be obtained in 3mm or 5mm vials from a pharmacy or online. Note that although it is a non-prescription item, some pharmacies may still require a prescription. I provide a preprinted, signed medical prescription to all of my scleral lens patients. This has two benefits: it tells the pharmacist that the solution is for scleral contact lenses (thus saving us both a phone call) and it increases the likelihood that the patient’s medical insurance will cover the expense.5,6
Scleral patients suffering from dry eye and those whose lenses exhibit areas of touch or minimal clearance may benefit from filling their lenses with unit-dose artificial tears instead, which provide extra lubrication and corneal protection. Keep in mind, however, that only the clear brands, not the milky or viscous ones, will work without compromising visual clarity. Also, try to avoid formulations with HP-GUAR; while a fantastic wetting agent, it has the potential to gel underneath the lens.7
| Generic Solutions, Specific Problems Every year or so, a retailer entertains bids on which company will produce and package its private-label solution. When the contract expires, the formulation may likely change. Because a retailer generally accepts the lowest bidder, companies typically do not place their premium solutions in generic bottles. So, because expiration dates are typically 18 to 36 months into the future, there could be two different chemical formulations residing in two of the same bottles sitting side-by-side on the store shelf. Likewise, a retailer can label two bottles of the same formulation differently—each to mimic popular brands.15,16 The bottom line: older formulations were the state-of-the art 10 or 15 years ago, and most work well for most patients. But since then, we have learned more about material/solution interaction; the private label solution may not be what’s most compatible for the patient.17,18 Research has also demonstrated a statistically higher rate of such ocular complications with patients who use private label compared with name-brand solutions.19 Additionally, FDA recommended in 2010 that all multipurpose solutions carry “rub and rinse” instructions, and the care systems launched since then have complied. However, some mass retailers’ packages still contain the words “no rub.” It has been common knowledge for several years that a cleaner lens is a healthier lens, and research has proven that digitally rubbing a lens is more effective than not at removing the deposits that can lead to inflammation or infection.20 We have made great advances in solutions over the past few years—including improved cleaning and disinfection, less toxicity, and increased comfort and wettability—and the patient who buys generic isn't benefiting from that technology.16 |
Manufacturers are also increasingly recommending against use of larger (e.g., 4 oz.) bottles of nonpreserved saline because they often contain buffers, which can contribute to debris or mucin buildup underneath the lens.8,9 Patients are also less likely to comply with discarding a larger bottle should it become contaminated.
Note that all of the options currently available for filling scleral lenses are considered off-label by the US Food and Drug Administration. That being said, research may one day produce a solution that is more biocompatible and similar to the tear film, but for now, unit-dose nonpreserved saline is the best option we have at this time.5,6
Cleaning Scleral Lenses
Scleral contact lenses are simply large GP lenses, so any solutions approved to clean and disinfect corneal GP lenses can be used for scleral lenses. However, because there is less tear flow under the edge of a scleral lens compared with a corneal GP lens, additional care should be taken to ensure that the lens surface is both clean and free of pathogens. I recommend a separate daily cleaner for all scleral lenses, regardless of whether they’re plasma-treated or not.
Examples of daily cleaners suitable for GP lenses include: Boston Cleaner (Bausch + Lomb), Boston Advance Cleaner (Bausch + Lomb), Optifree Daily Cleaner (Alcon), or Optimum by Lobob ‘Extra Strength Cleaner’ (Lobob). The latter, or an isopropyl alcohol-based cleaner approved for GP lenses, may be preferable for cleaning high Dk materials, which may scratch more easily with more abrasive cleaners. I also follow the FDA’s recommendation to rinse with saline, rather than tap water, to remove all cleaner from the lens, due to the fact that all water contains some levels of bacteria, fungi and amoebae.11
Lens disinfection should be performed with a GP conditioning/disinfection solution such as Boston Advance Comfort Formula Conditioning Solution (Bausch + Lomb) or Boston Conditioning Solution (Bausch + Lomb) or with a GP multipurpose solution such as Boston Simplus Multi-Action Solution (Bausch + Lomb), Menicon Unique pH (Menicon), Optifree GP (Alcon), or Optimum C/D/S (Lobob). In addition, the Menicon Deluxe Care System (with Progent) is now approved for home use.
Many scleral lens fitters advise sensitive patients to rinse the lens with nonpreserved saline prior to application. While this removes any residual solution—including its preservatives—left over from the disinfection process, it can also diminish wettability. Hydrogen peroxide solutions like PeroxiClear (Bausch + Lomb) or Clear Care (Alcon) are good preservative-free alternatives; however, these solutions are FDA-approved for GP lenses only if they are digitally rubbed prior to disinfection. If necessary, larger cases that accommodate diameters up to 30mm can be obtained from online stores like the Dry Eye Shop. The catalytic neutralization disc is not included with purchase, however, so one needs to be transferred from the smaller case prior to use.12
Hybrid Lenses
Hybrid contact lenses are comprised of a GP center surrounded by a hydrophilic skirt. Examples include the Duette and UltraHealth (SynergEyes). Manufacturer guidelines advise patients who wear these lenses to digitally rub the lens, front and back, with a daily cleaner approved for silicone hydrogel soft lenses, then rinse off the cleaner with nonpreserved saline. For disinfection, manufacturer guidelines recommend Clear Care (Alcon), BioTrue (Bausch + Lomb), Renu fresh (Bausch + Lomb) or Complete Easy Rub (Abbott Medical Optics). It should be noted that UltraHealth, due to its vaulted design, needs to be filled with nonpreserved saline or artificial tears prior to insertion, then inserted with the head kept down similar to inserting a scleral lens.
For keratoconus patients who wear the KC and ClearKone (SynergEyes) hybrid lenses, preservative-based care systems should be avoided. Hybrid lens manufacturer guidelines recommend Clear Care or Oxysept Ultracare (Abbott Medical Optics).
For all hybrid lenses, a digital rubbing step is required, as they have a six-month replacement schedule. Since they contain a soft skirt, gas permeable solutions are contraindicated.
Soft Lenses for Keratoconus
There are now excellent soft contact lens designs used specifically to treat keratoconus. Because these lenses are custom-produced and last up to three months, a digital rubbing step is typically recommended. (Of specific note, Bausch + Lomb recommends rubbing its KeraSoft IC lens in between the fingers, rather than in the palm of the hand.13)
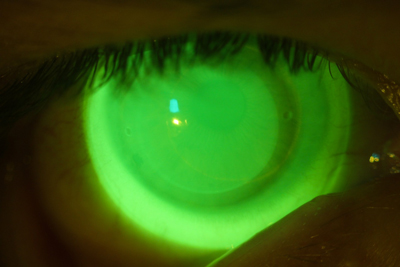 | |
| Fig. 3. Acceptable NovaKone (Alden Optical) fit on a keratoconic eye, enhanced with high molecular weight fluroescein. |
Because soft lenses for keratoconus tend to be thicker than disposable soft contact lenses, most manufacturers recommend using nonpreserved disinfectants because of the potential for absorption into the lens matrix. Alden Optical recommends hydrogen peroxide systems for its NovaKone lens (Figure 3), while Bausch + Lomb recommends use of either multipurpose or hydrogen peroxide with its KeraSoft IC. However, if multipurpose solution is used, B+L suggests rinsing it off with sterile rinsing solution prior to application.13
Conclusion
The fitting of scleral and other specialty contact lenses requires great diligence and attention to detail on the part of the practitioner. However, even the best scleral lens fit can be compromised by poor lens care on the part of the patient. This part of the equation is just as critical to contact lens success.
For further information on contact lens care, please consult each lens and/or material manufacturer for its specific care recommendations.
Dr. Gromacki is a Fellow of the American Academy of Optometry and a Diplomate in the Cornea, Contact Lens, and Refractive Technologies section. She has written extensively and lectured internationally on the topics of cornea and contact lenses and serves as the Director of the Contact Lens Service at a subspecialty group practice in Maryland.
1. Nichols, J. 2014 annual report: Contact lenses 2014. Contact Lens Spectrum. 2015;30(1):22-27.
2. Gromacki SJ. Handling and care of scleral GP contact lenses, Part 1. Contact Lens Spectrum. 2011;27(10):27.
3. Gromacki SJ. Scleral GP contact lens insertion, removal, and care. [Webinar.] Gas Permeable Lens Institute, March 2014. Available at: www.gpli.info/videos/webinar-2014-03.htm.
4. Dalsey Adaptives. The See-Green Lens Inserter. Available at: www.dalseyadaptives.com. Accessed February 2015.
5. Gromacki SJ. Handling and care of scleral GP contact lenses, Part 2. Contact Lens Spectrum. 2012;27(1):19.
6. Gromacki SJ. Scleral GP lens preparation: The latest standard of care. Contact Lens Spectrum. 2013;28(11):25.
7. Smythe M. Personal communication, April 10, 2014.
8. Imavasu M, Hori Y, Cavanagh HD. Effects of multipurpose contact lens care solutions and their ingredients on membrane-associated mucins of human corneal epithelial cells. Eye Contact Lens. 2010 Nov;36(3):361-6.
9. Gorbet MB, Tanti NC, Jones L, Sheardown H. Corneal epithelial cell biocompatibility to silicone hydrogel and conventional hydrogel contact lens packing solutions. Mol Vis 2010 Feb 19;16:272-82.
10. US Food and Drug Administration. Medical Devices. Available at: www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ContactLenses/. Accessed February 2015.
11. Ward M. General Session #9: Contact Lens Care. Lecture at The Global Specialty Lens Symposium, January 24, 2015; Las Vegas, Nevada.
12. The Dry Eye Shop. Lens Cases. Available at: www.dryeyeshop.com/lens-cases-c95.aspx. Accessed February 2015.
13. A&R Optical. Patient Instruction/Wearer’s Guide for Intelliwave3/KeraSoft IC. Available at: www.artoptical.com/files/documents/resources/Intelliwave_Patient_Instruction_-_Efrofilcon_A_1.pdf. Accessed February 2015.
14. Ichijima H, Shimamoto S, Ariwaka Y, et al. Compliance study of contact lens wearing in Japan, part 1: Internet survey of actual circumstances of lens use. Eye & CL 2014;40(3):169-174.
15. Ferris State University. Private Label Lens Care Guide. Available at: www.ferris.edu/HTMLS/colleges/michopt/vision-research-institute/PDFs/contact-lens-solutions.pdf. Accessed February 2015.
16. Gromacki SJ. The truth about generics. Contact Lens Spectrum. 2005;20(12):24.
17. Green JA, Phillips KS, Hitchins VM, et al. Material Properties That Predict Preservative Uptake for Silicone Hydrogel Contact Lenses. Eye Contact Lens. 2012 Nov;38(6):350-357.
18. Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses with multipurpose solution combinations. Optom. 2008; 79: 444-454.
19. Forister JY, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009;35(4):174-80.
20. Schnider C: Clinical performance and effect of care regimen on surface deposition of galyfilcon A contact lenses. [Electronic abstract 055102.] Optom Vis Sci. 2005; 82.


