The structural instability brought on by corneal ectasia and thinning makes keratoconus notoriously difficult to manage long-term, as the cornea becomes—literally—a moving target. Visual fluctuations and Rx changes in response to corneal shape change are common.
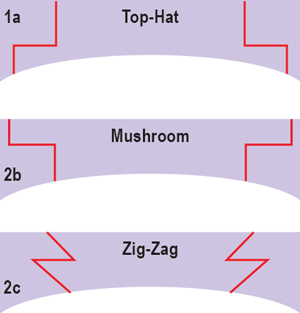 | |
| Fig. 1. From top to bottom: schematic of “top-hat” incision; schematic of “mushroom” incision; and schematic of “zig-zag” incision. |
Treatments for keratoconus have significantly advanced and diversified since the disease was first called to attention by Burchard Mauchart in 1748.1 First-line solutions include spectacles and contact lenses, with one caveat: soft lenses may be adequate to correct both myopic and astigmatic errors early in the disease process, but rigid gas permeable lenses will often become necessary as it progresses and corneal irregularity increases. Newer customizable lenses—such as sclerals, Rose K or Boston lenses—offer higher oxygen permeability and better comfort for these patients.2,3 However, in some cases, simple vision correction methods may not always be adequate. What other choices do patients have then?
Curtailing Progression
Ultraviolet A riboflavin-mediated corneal collagen crosslinking (CXL) may help prevent keratoconic disease progression in patients who exhibit a clear central cornea and minimum corneal thickness of 400μm. This surgical technique involves the biomechanical strengthening of the collagen fibers of the cornea by increasing the collagen crosslinks. Evidence for its efficacy and safety continues to be reviewed.4,5 Available globally since the late 1990s, the procedure has just received FDA clearance for use in the US.
Other surgical interventions should be considered when corneal irregularity is so great that spectacle or contact lens wear is no longer tolerated, when central scarring is limiting vision, or when significant corneal thinning may preface a possibility of traumatic perforation (a rare but devastating potential consequence of steroid use). Other FDA-approved surgical options include intrastromal corneal ring segments (ICRS) and corneal transplantation.
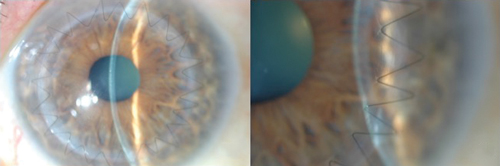 | |
Fig. 2. Left: A one-month postoperative slit lamp photo demonstrating a 24-bite running 10-0 nylon suture technique with zig-zag incision. Right: High magnification of graft/host interface with running suture demonstrating smooth surface transition at one month following zig-zag incision. |
ICRS are minimally invasive plastic polymer arc-shaped segments inserted into the cornea of keratoconus patients with mild cases of irregular astigmatism and preserved corneal thickness. Successful implantation requires a 6mm diameter zone of central cornea that measures more than 450μm in thickness. The ring segments are inserted into a pocket that can either be made manually or with a femtosecond laser. The incision is made based on the steep axis, as determined by corneal topography.
The goal of ICRS is to reduce corneal steepening as well as irregular astigmatism, so that patients can use contact lenses or spectacles with improved tolerability.6 Long-term research has demonstrated improvements in corrected distance visual acuity (CDVA) and spherical equivalent and a reduction of topometric astigmatism over the first few years in 60% of patients. Note, there does exist a possibility for clinical regression over the long term, as this treatment modality does nothing to blunt the progression of the disease, based on patients followed over five years.7 Currently, ongoing studies are evaluating the potential benefit of combining ICRS with corneal crosslinking as ultimately—even after ICRS implantation—the patient’s disease may continue to progress and eventually require corneal transplantation.8
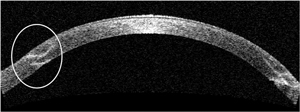 | |
| Fig. 3. OCT scan of a zig-zag incision taken one month post-op shows good alignment of donor and host tissue at both the anterior and posterior surfaces. | |
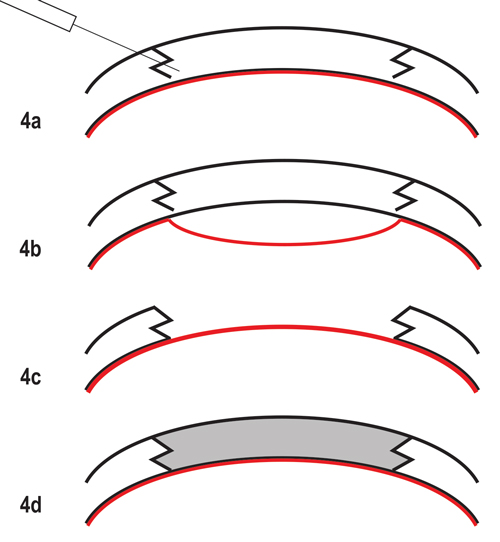 | |
| Fig. 4. The fs-DALK graft technique. (a) Baring of Descemet’s (highlighted in red) using “big bubble” technique after standard non-penetrating femtosecond laser zig-zag incision. (b) “Big bubble” technique with bubble dissecting tissue anterior to Descemet’s membrane. (c) Removal of entire stroma, preserving host DM and endothelium. (d) Suturing of donor tissue after donor endothelium has been removed. |
Advanced Stage Options
Patients with more pronounced presentations of keratoconus who are unable to tolerate contact lenses, who have significant scarring on the central visual axis or who fail to improve in visual acuity with corneal crosslinking or ICRS may require keratoplasty.
The choice of employing lamellar vs. full-thickness keratoplasty will depend on the extent of corneal scarring. Types of corneal transplantation techniques include conventional full-thickness penetrating keratoplasty (PKP), deep anterior lamellar keratoplasty (DALK) and femtosecond-enabled keratoplasty (FLEK), which can be performed as either a full-thickness graft procedure or in combination with the DALK procedure (known as fs-DALK).
In conventional full-thickness PKP, manual trephination can lead to misalignment of host and donor tissue, which may ultimately heal in such a way as to limit visual outcome.9 Patients most likely to benefit from a conventional PKP rather than a FLEK operation include those who possess a mechanical issue like a history of glaucoma implant surgery with bleb, or anterior staphyloma that prevents the laser interface from having direct contact with the corneal limbus due to the irregular ocular surface. As such, contraindications to FLEK include a history of glaucoma filtering surgery, the presence of an aqueous drainage device or narrow palpebral fissures.
The advent of the femtosecond laser has allowed for greater precision of wound architecture in both the host and donor tissue, allowing for quicker healing of the incision, improved approximation of tissue and quicker visual recovery with FLEK.10 During the procedure, the femtosecond laser pushes microcavitation bubbles to a precise corneal depth through photodisruption. These bubbles combine in a manner that results in planar cuts of tissue, a technique that was originally pioneered for use in laser in-situ keratomileusis (LASIK) flap creation.9 Various configurations for keratoplasty incisions have been designed with the femtosecond laser, including but not limited to “top hat,” “mushroom” and “zig-zag” (Figure 1).
Evidence has demonstrated these wound configurations allow more surface area for tissue healing—thus decreasing rates of wound leakage and enabling faster visual recovery—lower levels of postoperative astigmatism and earlier removal of sutures (Figure 2).11-17
Although no head-to-head comparisons have been conducted between the various configurations of wound architecture, the zig-zag-shaped incision appears to have some advantage over the others, as it leaves room for a smoother graft/host interface transition as well as decreased vertical and torsional misalignments due to its ability to create a hermetic wound seal (Figure 3).10
Regarding FLEK—an alternative to conventional PKP—some studies have demonstrated an improvement in CDVA, spherical equivalent and topographic astigmatism. Additionally, the advanced precision provided by the femtosecond laser allows for adequate scarring at four months post-op, creating the opportunity for possible early suture removal and topical steroid tapering.8
The deep anterior lamellar keratoplasty surgical approach to anterior corneal disease allows for selective replacement of diseased corneal epithelium and stroma after separation from healthy host Descemet’s membrane and endothelium. DALK is beneficial for keratoconus in that it provides the safety of extraocular surgery, the elimination of endothelial rejection, the potential for shorter post-op steroid regimens and increased graft longevity.18 Of note, stromal rejection is still a risk and should be routinely monitored for.
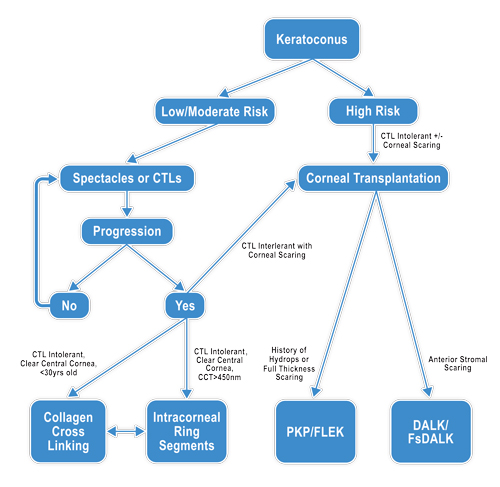 | |
| Fig. 5. Surgical management of keratoconus decision tree. |
The DALK “big bubble” technique, combined with the femtosecond laser, was first described in 2009.19,20 Femtosecond DALK offers the advantages of FLEK, including better donor/host fit, increased surface area apposition and faster wound healing, as well as the inherent benefits of DALK in treating stromal and anterior corneal disease (Figure 4).21-23 Additionally, fs-DALK is easier to perform than manual DALK because surgeons have the ability to customize the posterior ablation depth, allowing for more accurate dissection just anterior to Descemet’s membrane and reducing risk for perforation. Since a presentation of corneal hydrops can involve the Descemet’s membrane, keratoconus patients with a history of hydrops are not good candidates for DALK; instead, full-thickness grafts are recommended in these cases. Additionally, extreme corneal thinning increases the risk for Descemet’s membrane perforation, requiring intraoperative conversion of fs-DALK to a full-thickness keratoplasty. Fortunately, doing so maintains the advantages of femtosecond laser incision.19
There is a lack of reported data on the best surgical option at any given stage of keratoconus; as such, ultimately, the clinician must decide based on exam findings, patient symptoms and disease state (Figure 5). As always, initial management should first involve spectacles and/or contact lens wear. Corneal crosslinking may play a role in stabilizing disease progression, but further research is needed to determine efficacy and safety. ICRS can decrease refractive error and irregular astigmatism. Corneal transplantation is reserved for more advanced disease; however, patient outcomes have been vastly improved by recent improvements in technology and technique. The advent of the femtosecond laser has given surgeons a new tool for achieving better and faster visual recovery. Future options may also include novel artificial or engineered corneas for keratoplasty.
1. Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998;42:297-319.
2. Rathi VM, Mandathara PS, Dumpati S. Indian J Ophthalmol. 2014 Aug;61(8):410-5.
3. Barnett M, Mannis MJ. Contact lenses in the management of Keratoconus. Cornea. 2011 Dec;30(12):1510-6.
4. Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009;35:621–4.
5. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet – A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801.
6. Torquetti L, Berbel RF, Ferrara P. Long-term follow-up of intrastromal corneal ring segments in keratoconus. J Cataract Refract Surg 2009;35:1768-73.
7. Vega-Estraga, A Alio, JL Plaza-Puche AB. Keratoconus progression after intrastromal ring segment implantation in you patients: Five-Year follow-up. J Cataract Refract Surg 2015;41:1145-52.
8. Shetty R, Kaweri L, Pahuja N, et al. Current review and a simplified “five-point management algorithm” for keratoconus. Indian J Ophthalmol. 2015 Jan;63(1):46-53.
9. Farid M, Steinert R, Garg S, Wade M, et al. Cornea: Fundamentals, Diagnosis, and Management. Vol 2. 4th ed. Philadelphia: Elsevier Mosby.
10. Farid M, Steinert RF, Gaster RN, et al. Comparison of penetrating keratoplasty performed with a femtosecond laser zig-zag incision versus conventional blade trephination. Ophthalmology. 2009 Sept;116(9):1638-43.
11. Ignacio TS, Nguyen TB, Chuck RS, et al. Top-hat wound configuration for penetrating keratoplasty using the femtosecond laser: a laboratory model. Cornea 2006 Apr; 25: 336-340.
12. Steinert RF, Ignacio TS, Sarayba MA. Top-hat shaped penetrating keratoplasty using the femtosecond laser. Am J Ophthalmol 2007; 143(4):689-91.
13. Farid M, Kim M, Steinert RF. Results of penetrating keratoplasty performed with a femtosecond laser zigzag incision: initial report. Ophthalmology. 2007;114(12):2208-12.
14. Buratto L, Bohm E. The use of the femtosecond laser in penetrating keratoplasty. Am J Ophthalmol. 2007;143(5):737-42.
15. Price FW, Price MO. Femtosecond laser shaped penetrating keratoplasty: one-year results utilizing a top-hat configuration. Am J Ophthalmol. 2008; 145(2):210-14.
16. Cheng YY, Tahzib NG, van Rij G, et al. Femtosecond laser-assisted inverted mushroom keratoplasty. Cornea. 2008; 27(6):679-85.
17. Bahar I, Kaiserman I, Lange AP, et al. Femtosecond laser versus manual dissection for top-hat penetrating keratoplasty. Br J Ophthalmol. 2009; 93(1):73-8.
18. Reinhart WJ, Musch DC, Jacobs DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. Jan 2011;118(1):209-18.
19. Farid M, Steinert RF. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. J Cataract Refract Surg. 2009;35:809-13.
20. Price FW, Price MO, Grandin JC, et al. Deep anterior lamellar keratoplasty with femtosecond-laser zigzag incisions. J Cataract Refract Surg. 2009;35:804-8.
21. Lu Y, Shi YH, Yang LP, et al. Femtosecond laser-assisted deep anterior lamellar keratoplasty for keratoconus and keratectasia. International journal of Ophthalmology. 2014;7(4):638-43.
22. Buzzonetti L, Petrocelli G, Valente P. Femtosecond laser and big-bubble deep anterior lamellar keratoplasty: a new chance. Ophthalmology. 2012;2012:264590.
23. Shehadeh-Mashor R, Chan CC, Bahar I, et al. Comparison between femtosecond laser mushroom configuration and manual trephine straight-edge configuration deep anterior lamellar keratoplasty. British Journal of Ophthalmology. Jan 2014;98(1):35-9.


