Eye disorders are widely prevalent, with an estimated 3.5 million Americans age 40 years or older exhibiting some form of impaired vision. Though some of these are rare inherited conditions, more common issues make up a large segment of the group. Allergic conjunctivitis affects approximately 40% of the population yearly, including an increase in incidence over the last decade.7 Meanwhile, anterior ocular inflammatory diseases (AOID) like ocular allergy, dry eye and tear film dysfunction can present as comorbidities in patients with bacterial or viral infections.6 Clinicians are actively aware of the mechanisms that cause symptoms, yet many of these cases remain hard to treat.
Currently, a significant amount of research exists with regards to decreasing ocular inflammation from ocular allergies, infections and dry eye. Multi-drug therapies target the mediators responsible for clinical signs and symptoms, while mast-cell stabilizers combined with antihistamines enhance treatment effects throughout the body. The purpose of this article is to discuss the spectrum of receptors responsible for maintaining the homeostatic environment by modulating the innate and adaptive immune system.8
Passing Go
When an initial allergen penetrates through the physical barriers of the body, antigen-presenting cells (APC) grab it and migrate to the nearest lymph node, where they bind to the T-helper cells present there. The bound B-cell then awaits a cytokine signal from the Th2 cell in the form of interleukin IL-4. This signal triggers the formation of IgE-antibodies specific to the allergen within the B-cell that flow throughout the body, binding to mast cells that prompt a release of histamine and leukotrienes in response. Th2 helper cells also release cytokines, activating both basophils and eosinophils, which produce mediators to assist with the release of histamines and leukotrienes. This further amplifies the body’s allergic response; histamine release in particular accounts for many of the symptoms attributed to allergic conjunctivitis.
Following primary exposure, the allergens present in the body bind to the IgE on mast cells, triggering a release of mediators and IL-4, which causes the B-cells to produce more IgE antibodies in a positive feedback loop.9 Understanding this allergen pathway allows clinicians to target specific cells and mediators to control symptoms.
Allergic conjunctivitis constitutes a range of allergic inflammatory disorders that affect the anterior surface of the eye—namely, acute, seasonal and perennial conjunctivitis, and chronic allergic forms like atopic keratoconjunctivitis, vernal conjunctivitis and giant papillary conjunctivitis.1-3,6,7 Stored mediators like histamine and de novo chemicals (including derivatives of the arachadonic acid cascade comprising of leukotrienes and prostaglandins) initiate the acute phase reaction, while the late phase response is initiated by cytokines that facilitate the infiltration of eosinophils.10 The late phase is also the basis for chronic allergic disorders, with remodeling that involves stimulation, activation and localization of lymphocytes within the follicles and papillae of the conjunctival surface. Acute presentations of allergic conjunctivitis can be controlled with different topical ocular agents, while more severe forms may require a multidisciplinary approach in partnership with an ophthalmologist.3,10
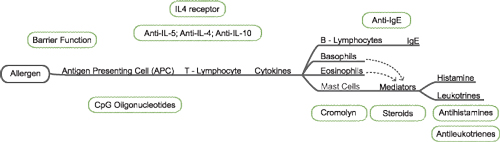 | |
| Fig. 1. Treatment path of ocular allergy. Source: Leonard Bielory, MD. Reproduced with permission of the author. |
Comparing the Early and Late Responses
An examination of the tear fluid content of an affected patient typically reveals the presence of a “soup” containing various preformed and de novo mediators like IgE antibody, histamine, leukotrienes and tryptase.10 Preformed mediators like histamine, tryptase and bradykinin immediately disperse following exposure to an allergen, while release of de novo mediators like leukotrienes and prostaglandins peaks at roughly eight to 24 hours following exposure. Cytokine release helps mediate both early- and late-phase responses to the allergen; these are direct contributors to the signs and symptoms of AOID. Early responses to allergen exposure typically present as pruritus and conjunctival erythema, which results from the release of histamine.3,7,10 Due to the direct relationship between early symptoms and histamine, early-phase reactants tend to respond best to antihistamine therapy for symptom relief.10
The allergic late phase, in contrast, is a delayed response without sustained exposure to the allergen. This phase involves a greater inflammatory response than the early phase, and is not primarily the result of histamine; this characteristic in fact contributes to the ineffectiveness of antihistamine therapy in patients with chronic allergic diseases.3,10 Unlike the immediacy of the early phase, the late phase can occur months after the initial allergen exposure and is marked by the recruitment of immune cells (i.e. dendrocytes, basophils, and eosinophils) to the site of the allergic response. Symptoms of late phase allergic responses include edema, pruritus, erythema, excessive tearing and lid and conjunctival edema. If corneal involvement is present, photophobia may also develop.
Dual action H1 subtype antihistamines in conjunction with mast cell stabilization are much more effective in combating symptoms of late-phase allergic reactions; however, the best available therapy for late phase symptoms may be steroids like loteprednol. Due to their ineffectiveness in the early phase and ocular side effects, however, steroids should not be considered as first-line therapy.1,2,7,10
Antihistamines act as antagonists to histamine receptors, inhibiting the release of acute-phase allergic reactants.6,7 Many antihistamines differ in their ability to treat specific AOID symptoms due to their varying affinities for each subtype of histamine receptor. Symptomatic relief is directly correlated with a clinician’s ability to find the antihistamine that targets the right receptor, and, since mast cells are responsible for the late-phase allergen response, the role of antihistamines and their relative potency are extremely valuable to patient care. Comparing the binding affinity of each antihistamine to each of the four subtypes of histamine receptors can help a clinician choose a more targeted therapy plan for the patient.2,3,7
The ability to bind with histamine receptors is based on the rate the drug binds to receptors, the duration of binding and the maintenance of the drug-receptor complex. These factors combine to enable the drug’s ability to antagonize the activation of histamine receptor to achieve symptomatic control and minimize side effects.3,7 Commonly, antihistamines work by binding to the active site of receptors, leading to the inactivation of histamine. Inhibition of histamine receptors is achieved when enough of the drug is present to prevent activation of the receptor. Drugs that have poor binding affinities require higher concentrations to achieve the same inhibition, compared to drugs with a higher binding affinity. As such, a drug’s affinity for a targeted histamine receptor can be used to predict how potent its antihistamine effects will be.3,7
Drug vs. histamine binding at the receptor is a competitive process, as both the drug and histamine bind to and release from the receptor. As such, the compound that spends the most time bound to the receptor will cause the receptor to be stimulated or inhibited. Efficacy of antihistamines can be greatly enhanced by rapid equilibrium and onset of action, or by slow dissociation rates from the receptor.3,6,7,10 Note, however, antihistamines carry an anti-cholinergic effect that can cause ocular drying. This side effect is the result of muscarinic receptor inhibition. In some cases, however, it can be used for the treatment of insomnia or excessive allergic rhinorrhea. Thus, it is important to understand binding affinities for muscarinic receptors of current antihistamines to prevent adverse side effects without affecting the efficacy of the treatment provided.1-3,6,7
Histamine Receptor Subtypes
Histamine is considered both an autacoid and a neurotransmitter; nearly all organs and a wide range of biological functions are affected by it. These effects are mediated through the distribution of four types of G-coupled proteins. Histamine binds to receptors throughout the body based on location of the necessary receptors and the physiological response that triggered its release. All histamine receptors exhibit constitutive activity, however, and are able to function without bound histamine. Research has also indicated that H1 and H2 receptors are more widely expressed than H3 and H4 receptors.7,9,10
Histamine receptors are triggered via a second messenger system associated with G-coupled proteins. All histamine receptors are heptahelical transmembrane molecules that transduce extracellular signals by way of G proteins to intracellular second messenger systems. These include Ca2+, cGMP in H1, cAMP in H2, Ca2+ and MAP kinase for H3 and H4 receptors. Different cell types and their physiological locations give histamine its ability to trigger varying effects based on its target receptor.7-10
With respect to the four subtypes described in Table 1, H1 receptors are predominately located in the bronchi, heart, central nervous system (CNS) and arterial and intestinal smooth muscle.7,9 The sedative effect of H1 antagonism seen in first generation oral antihistamines, (i.e., diphenhydramine) is the result of their ability to cross the blood-brain barrier, while the appearance of histamine receptors in smooth muscle contributes to the cardiac side effects present in some oral antihistamines.2,7 H1 stimulation is associated with inflammation, prostaglandin production, headache, hypotension, tachycardia, and bronchoconstriction. H1 is the only histamine responsible for pain, and is also the subtype most contributory to itching. Research has indicated H1 antagonism prevents cytokine release and ocular itching associated with allergic conjunctivitis, and also increases vascular permeability.6,7,10
| Table 1. Histamine Receptor Subtypes7 | ||||
| H1 | H2 | H3 | H4 | |
| Immunomodulatory effects | X | X | X | X |
| Itching | X | X | ||
| Swelling | X | X | ||
| Erythema | X | X | ||
| Vascular permeability | X | X | ||
| Pain | X | |||
| Vasodilation | X | |||
| Nasal congestion | X | |||
H2 receptors are located in the parietal cells of the gastric mucosa, CNS, uterus and the heart. Drugs like cimetidine and famotidine inhibit this receptor’s role in gastric acid secretion. H2 receptors cause increased vascular permeability, flushing and relaxation of bronchial smooth muscle relaxation. H2 receptors also have a vasodilatory effect and have been shown to prevent stimulated vasodilation with prior administration of H2 antagonists.7,10
H3 receptors are present in neurons throughout the CNS and peripheral nervous systems, as well as in the heart and bronchioles. H3 receptors are located pre-synaptically, and their stimulation enhances modulation of the blood-brain barrier while inhibiting release of histamine and other neurotransmitters. H3 receptors are responsible for bronchial relaxation, inhibition of sympathetic neurotransmission, decrease in gastric acid production and control of vasoactive mediators. These effects are kept in mind when targeting H3 receptors to control insomnia, obesity, inflammatory diseases, schizophrenia and certain disease states mediated by neurotransmitter release.7,10
H3 receptor antagonism has been shown to aggravate pruritic symptoms, which appears to contradict the normal histamine-induced pruritus pathway. It was found to increase the incidence of scratching behavior in mice; further research indicated intradermal injections of an H3 receptor antagonist (lodophenpropit or clobenpropit) triggered a significant increase in scratching behavior in both mast-cell deficient and wild type mice. H3 antagonism-induced pruritus appears to be mediated by substance P, a known pruritus-causing agent. Some speculation has occurred that clobenpropit induced a pruritic response through H3 subtype antagonism in combination with H4 subtype agonist.6,7,9,10
As such, H3 blockade may provide relief of nasal congestion symptoms. Research supports the opinion that H3 receptors are involved with sympathetic transmission, and that combined H1 and H3 antagonism can provide treatment for allergic rhinitis symptoms. This is useful in patients that continue to be symptomatic when only using an antihistamine as a treatment regimen. Combination H1 antagonism (chlorpheniramine) and H3 antagonism (clobenpropit or thioperamide) revealed significant decongestive effects, while evading hypertension. Further research into combining H1 receptor antagonist (fexofenadine) with a novel H3 antagonist found statistically significant relief of subjective symptoms like rhinorrhea, itching and sneezing compared with the placebo.3,4,7
The H4 receptor is directly related to pruritus as well. It is expressed on hematopoietic cells and plays a crucial role in the function of mast cells, eosinophils, monocytes, dendritic cells and T-cells. Studies have identified that the chemotactic response of mast cells, eosinophils, dendritic cells, monocytes and natural killer cells to histamine is through the H4 subtype receptor.10 These receptors are involved in autoimmune reactions, allergies and, specifically, symptoms of pruritus. The immunomodulating effects of the H4 subtype receptor are currently under investigation for its uses in anti-allergy therapy. H4 receptor antagonism is also being investigated for treating immune-related diseases like asthma. H1 receptor antagonism alone has been shown to be ineffective in the treatment of asthma, but in combination with H4 receptor blockade may provide symptomatic relief.2,3,6,7,8,10
H4 subtype antagonism has been studied in a long-term experimental murine model of pruritus associated with a chemical-induced contact dermatitis induced by repeated challenge with 2,4,6-trinitrochlorobenzene [TNCB].10 H4 antagonist [JNJ7777120] was administered and shown to reduce pruritus and resolve skin lesions in a dose-dependent manner. Compared to when managing H1 receptor antagonism with fexofenadine, however, relief was not seen in both inflammation and pruritus.7 It was also demonstrated that H4 receptor agonists elicited a scratching response that can be ameliorated by pretreating with a selective H4 receptor antagonist, and that when H1 and H4 subtype receptors are both antagonized, scratching behavior was nearly completely relieved. Pruritic symptoms were also brought under control with H1 antagonism in H4 subtype receptor knockout mice.6,7,10
Murine models have hypothesized the concept of synergistic control of symptoms relating to allergic disease states. Studies have shown that in murine models with induced pruritus, there is statistically increased symptomatic control when H1 and H4 receptor antagonists were used concurrently. It was also noted that any pruritus in the H4 knockout mice was resolved with administration of diphenhydramine.7
| Table 2. Binding Afinity [Ki] Comparison | ||
| H1 Receptor [nM] | H2 Receptor [nM] | |
| Olopatadine | 35.0nM | 1,000,000nM |
| Azelastine | 6.8nM | 4,200nM |
| Ketotifen | 1.3nM | 1,000nM |
| Epinastine | 9.8nM | 4,030nM |
The Ties That Bind
Literature comparing the binding affinities of 19-marketed anti-histamines has indicated differences in receptor affinity and drug potency—namely, a larger binding affinity value [i.e., Ki] correlates with a weaker binding affinity of the drug for the receptor. If the binding affinity of the drug to the receptor is weak, a larger dose of the drug will be needed to achieve the same inhibition as a drug with a lower Ki.
Relative potency can also be determined using these Ki values. Antihistamines marketed for muscarinic receptor inhibition were compared for potency and possible trends in anti-cholinergic side effects; these studies of binding affinities were separate studies and were not in direct comparison, but some trends become apparent.6,7,10
Histamines on the Market Azelastine is a selective H1 receptor antagonist that also exhibits H2 blocking properties. Azelastine also blocks the release of histamine from mast cells, and formation of leukotrienes. It has a relatively low pH of 5.0–6.5, making its major side effect mild ocular irritation on administration. It is relatively long acting, being efficacious for up to eight hours when used twice daily and is available in an intranasal formulation as well.1,2,6,7,10 Epinastine is an antihistamine with a high affinity for H1 receptor and H2 receptor antagonism, with mast cell-stabilizing properties as well. Originally approved for rhinitis, with ocular administration it has shown significant improvement of itching, swelling and tearing compared with the placebo. It is recommended twice daily with duration of eight hours or longer. Side effects include burning and infection, and it is generally well tolerated for up to eight hours after instillation. Bepotastine is an H1 receptor selective antagonist with mast cell-stabilizing and eosinophil modulating properties. It is approved as an ophthalmic solution for the treatment of itching associated with allergic conjunctivitis. It was effective in controlling ocular itching and tearing, but shows little effect on conjunctival redness. Bepotastine has been shown to have rapid onset and can give symptomatic relief in as soon as three minutes after administration and last for up to eight hours with a statistically significant number of patients achieving complete relief. Bepo-tastineb besilate 1.0% was able to inhibit vascular permeability due to histamine blockade substantially better than olopatadine 0.1%. In addition to ketotifen, bepotastine has shown an ability to inhibit eosinophil infiltration into the conjunctiva.1,2,5-8 Alcaftadine is a tricyclic piperidine aldehyde approved for the treatment of itching associated with allergic conjunctivitis. It is administered once daily and demonstrates substantial relief of symptoms at both the 15-minute and 16-hour mark after administration. Both oral and ophthalmic formulations show very similar pharmacokinetic activity. Cytosolic enzymes via aldehyde oxidation metabolize alcaftadine to its acid metabolite. It is believed that cytochrome p450 enzymes play a minor role in the metabolism of the drug.6 Alcaftadine exhibits antagonistic activity on H1 and H2 subtype receptors, as well as low affinity for the H4 subtype. Furthermore, alcaftadine prevents the recruitment of eosinophils and inhibits mast cell degranulation. Treatment with alcaftadine has shown less eosinophil infiltration into the conjunctiva compared to olopatadine and the placebo in murine models. In direct comparison with other ocular allergy agents, alcaftadine has shown to provide similar relief to current marketable agents.3,6,7 |
The binding affinity for histamine to the H1 receptor is 1,80,000nM. Thioperamide has the lowest affinity for H1 receptor with a Ki of 2,80,000nM. Pyrilamine had a Ki value of 0.8nM, giving it the highest affinity for the H1 receptor of all the agents compared. The first-generation drug diphenhydramine is also a very potent binder, with a Ki of 12.5nM, while potency was variable with regards to the second-generation compounds. Desloratidine (Ki 4nM) and cetirizine (Ki 6.3nM) showed the highest affinity, while loratadine (Ki 35nM) and fexofenadine (Ki 83nM) had much lower binding affinities. Topical ophthalmic formulations have very high potency, with olopatadine (35nM) having the lowest affinity and ketotifen (1.3nM) and emedastine (1.3nM) having the highest affinity.7,10
H2 receptors have much weaker binding affinities compared to those of the H1 receptor.7 The binding affinities for H2 subtypes are two to four orders of magnitude weaker than those for the H1 receptor. Ranitidine showed the greatest affinity for the H2 receptor with a Ki of 187nM. Olopatadine [Ki 1,00,000nM] had the weakest binding affinity to the H2 receptor whereas diphenhydramine, azelastine, epinastine and ketotifen showed binding affinities in the range of cimetidine [Ki >10,000nM]. Using histamine’s affinity to H2 subtype receptors, we can model the drug’s ability to compete and produce the desired antihistamine affect. Histamine’s affinity to H2 subtype receptors is 18,350nM, but it can be inferred that drugs such as emedastine (Ki 49,067nM) and olopatadine would need much higher concentrations to overcome competition with histamine for the H2 receptor.1,2,7,10 H3 and H4 subtypes have more similarities between the two than the H1 and H2 subtypes. The highest affinity for H3 was thioperamide (Ki 79,400nM) and olopatadine (Ki 79,400nM) exhibited the lowest affinity for the H3 receptor.7
Available data on H4 receptor affinities is limited. Only five antihistamines were compared: thioperamide [Ki 27nM] had the greatest affinity while cimetidine and ranitidine [Ki >10,000nM] exhibited relatively low affinity for the H4 subtype receptor. More in-depth research into H4 receptors and their function must be conducted to determine the suitability of using H4 subtype agonists or antagonists for treatment of conditions as it relates to allergic and inflammatory disease processes.7,10
Due to the ability of some antihistamines to exert an effect on muscarinic receptors, antihistamines that offer the least amount of anticholinergic activity while maintaining high antihistamine potency would be ideal. Drugs such as desloratidine have shown the greatest effect on muscarinic receptors.1,2,6,7,10 Ketotifen is a strong H1 antagonist and mast cell stabilizer with leukotriene inhibition. It is the only topical ophthalmic agent available without a prescription in the United States. If administered twice daily, it has been found to be effective in the prevention of itching, redness and general symptoms of allergic conjunctivitis compared to the placebo. Emedastine difumurate was compared to ketotifen fumarate; both showed marked improvement in the incidence of itching compared with the placebo.1,2,6,7,8,10
The ever-increasing incidence of AOIDs suggests clinicians must continue to develop new, innovative treatments to alleviate patient symptoms. Of the four known types of histamine receptors, each has varied effects dependent on location and physiological signal. Additionally, each exhibits its own specific response to acute inflammation; thus, understanding each receptor’s unique and localized actions is key for targeting treatment. Persistent research relating to the affinity of antihistamines and the ability to target subtypes may also open the door for new allergic disease management techniques; as such, these may be the next step in managing AOIDs.
Dr. Brett Bielory was previously an Ocular Pathology Fellow at Bascom Palmer Eye Institute in Miami, Florida. He is currently a resident in ophthalmology with an interest in corneal and ocular surface diseases at New York Medical college in Valhalla, NY.
Dr. Steven Shah is a graduate of the Medical University of Lublin in Poland. He is currently working as a clinical research associate at the STARx Asthma and Allergy Center in Springfield, NJ.
Dr. Leonard Bielory is an allergist-immunologist in New Jersey who is affiliated with multiple hospitals in the area, including Robert Wood Johnson University Hospital and St. Barnabas Medical Center.
1. Bielory BP, OBrien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta Ophthalmol 2012; 90(5): 399-407.
2. Bielory L. Allergic conjunctivitis: the evolution of therapeutic options. Allergy Asthma Proc 2012; 33(2): 129-39.
3. Bielory L, Meltzer EO, Nichols KK, Melton R, Thomas R, Bartlett JD. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 2013; 34(5): 408-420.
4. Bielory L, Skoner DP, Blaiss MS, et al. Ocular and nasal allergy symptom burden in America: the Allergies, Immunotherapy, and Rhinoconjunctivitis (AIRS) surveys. Allergy Asthma Proc 2014; 35(3): 211-18.
5. Bielory L, Duttachoudhury S, McMunn A. Bepotastine besilate for the treatment of pruritus.” Expert Opinion in Pharmacotherapy 2013.
6. Syed BA, Kumar S, Bielory L. Current options and emerging therapies for anterior ocular inflammatory disease. Curr Opin Allergy Clin Immunol 2014; 14(5): 485-489.
7. Wade L, Bielory L, Rudner S. Ophthalmic antihistamines and H1-H4 receptors. Curr Opin Allergy Clin Immunol 2012; 12(5): 510-16.
8. Bielory L. Allergic conjunctivitis: the evolution of therapeutic options. Allergy Asthma Proc 2012; 33:129-39.
9. Kumar V, Abbas A, Aster JC. Robbins & Cotran Pathologic Basis of Disease. Ninth Edition, Elsevier Saunders, 2011.
10. Bielory L, Lien KW, Bigelson S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs 2005; 65:215-28.


