US Refractive Surgeons Now Ready to SMILE
A new refractive surgery option, Zeiss’s VisuMax small incision lenticule extraction (SMILE) procedure, recently received FDA approval. The SMILE procedure involves the creation of a disc-shaped lenticule in the stroma using a femtosecond laser, which the surgeon removes through a small incision—obviating both the creation of a flap and use of an excimer laser needed for LASIK.
“Since there is no flap, there are no flap related issues. There is also vastly less interruption of nerve fibers so one would anticipate less dry eye,” says John F. Doane, MD, of Discover Vision Centers in Kansas City, MO, a clinical investigator for Zeiss. “The refractive results are essentially the same. Interestingly, since the surgery is done in a vacuum—i.e., the corneal stroma that is not exposed to the atmosphere—the procedure is dose independent. We are just as predictable with a -1D as we are with a -10D, which is unique to all prior corneal refractive procedures.”
A recent study examined the five-year results of the first 56 eyes treated with SMILE for myopia in 2008-2009 using the 200kHz VisuMax.1 Researchers found a mean regression of −0.48D over five years, which is at least as good as the data of other procedures, they noted—the mean regression for LASIK is 0.63D to 0.97D after six to seven years.2,3 They conclude SMILE is an effective, stable and safe procedure for myopia and myopic astigmatism treatment.1 They also note that laser technology advances—the newly approved procedure uses a 500kHz femtosecond laser—translates into better outcomes, and newer studies report refractive outcomes superior to the 2008-2009 results.4,5
The FDA’s approval came on the heels of a study demonstrating positive visual acuity and refractive predictability outcomes for 336 eyes treated with the SMILE procedure, according to a Zeiss press release.6 The study found participants had stable vision six months post-procedure, and all but one subject had uncorrected visual acuity of 20/40 or better; 88% had uncorrected visual acuity of 20/20 or better.
The SMILE procedure is indicated for the reduction or elimination of myopia of -1.00 D to -8.00D, with ≤ -0.50D cylinder and MRSE -8.25D in patients at least 22 years old with documented stable refraction over the past year, Zeiss says.
“If worldwide acceptance foreshadows what will occur in the United States, we can expect quick uptake of the SMILE procedure,” Dr. Doane suggests. Over half a million procedures have been performed worldwide, Zeiss says.
1. Blum M, Täubig K, Gruhn C, et al. Five-year results of small incision lenticule extraction (ReLEx SMILE). Br J Ophthalmol. 2016;100:1192–5.
2. Zalentein WN, Tervo TM, Holopainen JM. Seven-year follow-up of LASIK for myopia. J Refract Surg. 2009;25:312–8.
3. Sekundo W, Bönike K, Mattausch P, et al. Six-year follow-up of laser in situ keratomileusis for moderate and extreme myopia using a first-generation excimer laser and microkeratome. J Cataract Refract Surg. 2003;29:1152–8.
4. Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology. 2014;121:822–8.
5. Blum M, Kunert KS, Gille A, et al. LASIK for myopia using the Zeiss VisuMax femtosecond Laser and MEL 80 Excimer Laser. J Refract Surg. 2009;25:350–6.
6. Zeiss. Zeiss receives US FDA Approval for VisuMax SMILE vision correction procedure, the latest advancement in laser eye surgery. Press Release. September 14, 2016. Available at www.zeiss.com/meditec/en_de/media-news/press-releases/us-fda-approval-visumax-smile-vision-correction-procedure-in-laser-eye-surgery.html.
Microbiota Protects Against P. aeruginosa Infections
Ocular microbiota plays a role in protecting against Pseudomonas aeruginosa–induced infections, a recent study suggests. The reasons for the common association between contact lens wear and P. aeruginosa–induced keratitis have been unclear. While the existence of ocular microbiota is not news, there has been a dearth of functional analyses to probe the significance of its role in ocular immunity.
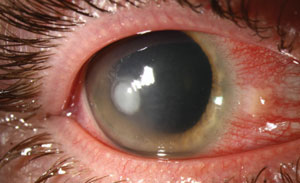 |
| Pseudomonas ulcers could be more likely in patients with compromised ocular microbiota, a new study says. Photo: Christine Sindt, OD. |
CL wearers, according to recent genomics-based approaches, harbor altered ocular commensal communities compared with non-lens wearers. This has given rise to important questions regarding the nature of contamination and the frequency of keratitis. More specifically, is this increased frequency of contaminated lens-induced keratitis a result of species deriving from the skin, or does ocular microbiota exert immune functions that are required for ocular health? Researchers found the presence of microbiota in healthy subjects served to strengthen the ocular immune barrier, resulting from microbiota’s role in increasing concentrations of immune effectors in the tear film.
Using Swiss Webster mice—which are typically resistant to P. aeruginsa-induced keratitis but become susceptible alongside a lack of microbiota—researchers demonstrated that commensal bacteria at the ocular surface serve to deliver regulatory signals regarding the scale of neutrophil recruitment during infection, according to a study in PLoS Pathology.
They posit that these events may result from coagulase negative Staphylococcus, a frequent gram-positive commensal. Further, the authors note that, in addition to the impact of ocular microbiota, gut microbiota may play a significant role in regulating the pool of mature neutrophils and their activation state. In fact, protective immunity was found to be dependent on both eye and gut microbiota, “with the eye microbiota having a moderate, but significant impact on the resistance to infection,” the study says. This work is the first to demonstrate microbiota as a regulatory agent regarding susceptibility to P. aeruginosa-induced keratitis. This previously unappreciated role of microbiota will hopefully help push forward more research to broaden the understanding of vulnerability and treatment regarding infections of the ocular surface.
Kugadas A, Christiansen SH, Sankaranarayanan S, et al. Impact of microbiota on resistance to ocular pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 12(9): e1005855.
In Brief
Conjunctival chemosis may be a marker to help clinicians predict Pseudomonas aeruginosa as the bacterial agent responsible for certain corneal ulcers, new research suggests. A masked review looked at 62 infective corneal ulcers and found 14 of 16 cases of P. aeruginosa–related corneal ulcers presented with conjunctival chemosis, compared with only six of the remaining 46 cases caused by other organisms.1 Whether or not chemosis was present predicted or excluded P. aeruginosa with roughly 87% accuracy, the researchers conclude.1
1. Michael KB, Rotchford A, Ramaesh K. Conjunctival chemosis as a specific feature of Pseudomonas aeruginosa corneal ulcers. Cornea. 2016;35(9):1182-4.
A new study sheds light on the impact environmental conditions have on tear inflammatory mediators in contact lens wearers.1 Fifty-four CL wearers were exposed to two environmental conditions: standard (50% relative humidity) or adverse (5% relative humidity). The researchers analyzed changes in concentration of: epidermal growth factor (EGF); interleukin (IL)-1 receptor antagonist, IL-1β, IL-2, IL-4, IL-6 and IL-8; tumor necrosis factor α; monocyte chemoattractant protein-1; and matrix metalloproteinase (MMP)-9. Under standard conditions, EGF increased significantly while IL-1β and IL-2 decreased significantly. Under adverse conditions, IL-6 increased significantly. During CL wear, secretion of several inflammatory mediators varies, depending on the type of CL and the environmental conditions at play, the study concludes.1
1. Martín-Montañez V, Enríquez-de-Salamanca A, López-de la Rosa A, et al. Effect of environmental conditions on the concentration of tear inflammatory mediators during contact lens wear. Cornea. 2016;35(9):1192-8.
Researchers recently evaluated the efficacy and safety of transcutaneous electrical stimulation for symptoms and clinical signs of dry eye. The study included 27 patients with dry eye who underwent transcutaneous electrostimulation, wherein electrodes were placed onto the periorbital region of both eyes in addition to manual stimulation with a hand-piece conductor.1 Results showed the Ocular Surface Disease Index improved from 43.0±19.2 at baseline to 25.3±22.1 at the completion of treatment.1 These improvements were maintained at six- and 12-month follow-up evaluations. As the study suggests it improves dry eye without adverse effects, transcutaneous electrical stimulation shows potential to widen the scope of treatment options for dry eye.
1. Pedrotti E, Bosello F, Fasolo A, et al. Transcutaneous periorbital electrical stimulation in the treatment of dry eye. Br J Opthalmol. September 4, 2016. [Epub ahead of print].

