Is the practice of instilling drops while concurrently wearing contact lenses acceptable? Let’s discuss the topic by analyzing two scenarios:
A 28-year-old female, recently fit in 30-day continuous wear monthly lenses, also occasionally suffers from mild ocular allergies. She does not want to remove her lenses each day to instill a once-daily anti-allergy medication.
A 72-year-old male wears two-week replacement extended wear monovision contact lenses, removing them one night per week for cleaning. His glaucoma requires drops twice a day. He does not want to alter his contact lens wear schedule to instill the drops.
Would you be able to accommodate these patients? How would you advise them? In this article, let’s take a closer look at the latest research on the concurrent use of ophthalmic medications and contact lens wear.
Contact Lenses and Drops
In some situations, continued contact lens wear can exacerbate the progress of a disease. In these cases, it is not appropriate to continue contact lens wear while using eye drops. The most obvious condition is infectious keratitis—of any form—including bacterial, herpetic, fungal and protozoal. Additionally, corneal trauma with an organic etiology would contraindicate contact lens wear. Ointments should typically never be utilized while wearing lenses either.
The use of a bandage contact lens in the management of recurrent corneal erosions or corneal abrasions often necessitates the instillation of ophthalmic drops while wearing a contact lens. Having the patient instill eye drops while wearing the contact lens is preferred over the potential of disrupting the healing epithelium and exacerbating the condition by removing the lens each time eye drops must be instilled. Pharmaceutical agents that may be utilized often include hypertonic solutions, lubricants, NSAIDs and prophylactic antibiotics.
Consider, for example, a patient who presented to our clinic with numerous metallic foreign bodies (see figure 1). After the removal of approximately 20 foreign bodies, extensive epithelial loss occurred. In order to enhance comfort, I inserted a therapeutic contact lens with a base curve of 8.7mm and plano power (Air Optix Night and Day Aqua, CIBA Vision). In this case, it would be possible to manage this patient pharmacologically with two preservative-free agents: Acuvail (ketorolac 0.45%, Allergan) in unit dose vials to be used b.i.d. and Vigamox (moxifloxacin 0.5%, Alcon) to be used t.i.d. I decided to treat this patient with Nevanac (nepafenac 0.1%, Alcon), Vigamox and preservative-free artificial tears—all t.i.d. for five days. Nepafenac contains benzalkonium chloride (BAK) 0.005%; the patient showed no adverse reactions including no corneal toxicity and was completely re-epithelialized in three days.
Ocular Sensitivity to Preservatives
A contact lens increases the residence time that the ophthalmic drop is in contact with the ocular surface. While there are some advantages to an extended delivery system, if the drug itself or one of the inactive excipients (e.g., the preservative) is irritating to the ocular surface, this increased contact time will only exacerbate the detrimental effect.
The FDA requires that multidose topical ophthalmic medications be preserved and ophthalmic medications have contained preservatives since the 1940s. All effective preservatives are toxic. Preservatives used in ophthalmic preparations include BAK, chlorhexidine, thimerosal, chlorobutanol, methyparaben/propylparaben and ethylenediaminetetraacetic acid (EDTA).
Newer preservatives include stabilized oxychloro complex (Purite [Allergan] and Ocupure [Abbott Medical Optics]), sodium perborate (GenAqua [CIBA Vision]), boric acid, propylene glycol, sorbitol and zinc chloride (sofZia [Alcon]). These preservatives are said to be disappearing: upon exposure to light or tears, they are converted into non-toxic compounds.
BAK and the Ocular Surface
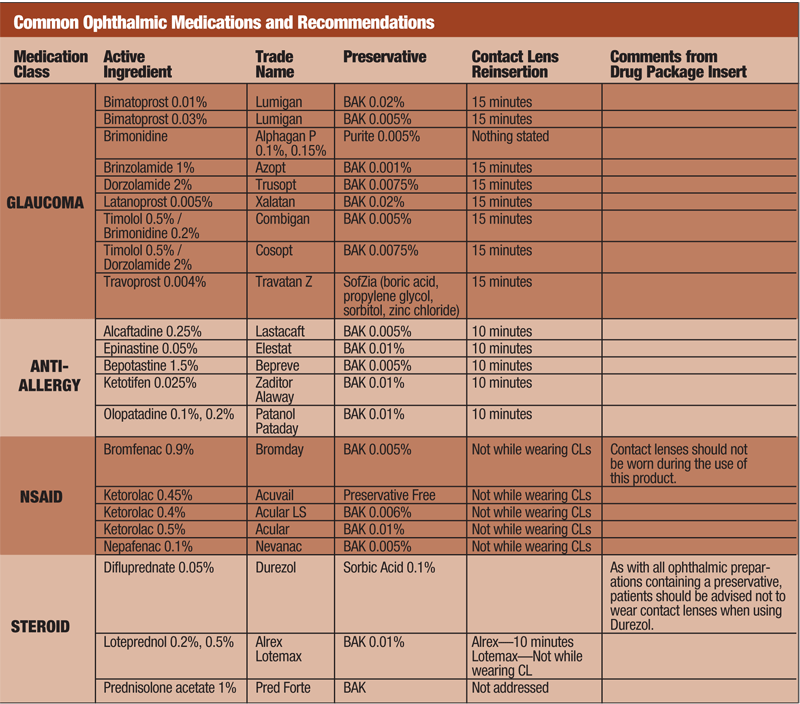
BAK is a quaternary ammonium compound, considered a detergent preservative. It alters cell membrane permeability causing the cell to rupture resulting in its bactericidal effect. BAK is by far the most common preservative found in today’s ophthalmic medications and is estimated to be present in 72% to 78% of eye drops (see “Common Ophthalmic Medications and Recommendations”). It is formulated in solutions at a concentration ranging between 0.004% and 0.02%; compared to other preservatives, BAK takes significantly less time to kill bacterial cells.1
Using transepithelial electric resistance on live rabbits, Mao Kusano, M.D., and colleagues showed that higher concentrations of BAK solutions (0.02%) disrupted the corneal epithelium within seconds.2 This phenomenon was less noticeable with lower percentages of BAK. Other preservatives—boric acid, chlorhexidine, chlorobutanol, EDTA and paraben—that were evaluated did not affect cell viability.2
Because the utilization of glaucoma medications is usually long term, the ocular tolerability to these agents and the preservatives formulated with them have been studied extensively. Bernard McCarey, Ph.D., and Henry Edelhauser, Ph.D., compared the effects of Travatan Z (travoprost, Alcon), preserved with sofZia, to Xalatan (latanoprost, Pfizer), preserved with 0.02% BAK, on the corneal permeability in rabbits.3 Treatment calls for one drop to be instilled every 60 seconds for three minutes. In a different study, these same researchers looked at a three-minute exposure to the drugs.3 There was a significant difference in epithelial toxicity, as demonstrated by increased epithelial permeability of corneas treated with latanoprost. Travoprost performed similarly to those corneas only treated with a balanced salt solution.
Using a monolayer of transformed human corneal epithelial (HCE-T) cell cultures, ocular epithelial cells were evaluated after a 25-minute exposure to various prostaglandin analogue solutions containing three different preservatives.4 Vehicles containing sofZia and BAK were tested independently to determine if the active drug had any effect on corneal health. BAK was found to cause significant cytotoxicity.4 The researchers emphasize the need for more work and despite the observed toxicity, the clinical significance of the difference between the preservatives has not yet been established.4
Malik Kahook, M.D., and Robert Noecker, M.D., looked at corneal morphology of rabbits exposed to travoprost/sofZia or latanoprost/BAK.5 They used a rabbit model to demonstrate goblet cell loss after 30 days of once daily instillation of latanoprost/BAK compared to once daily travoprost/sofZia.6 Latanoprost induced superficial cell loss likely due to the relatively high concentration of BAK contained within this medication.
Sei Yamazaki, M.D., and colleagues studied a group of patients who were switched from latanoprost/BAK to travoprost/sofZia because of superficial punctate keratopathy (SPK).7 These patients were assessed at baseline and then at two weeks and at the first, second and third monthly increments. SPK was found to improve at all time points; the absence of BAK was attributed to the improvement.7
These findings contradict the results from a study by Jess Whitson, M.D., and colleagues who compared the ocular tolerability of the same two prostaglandins and Lumigan (bimatoprost 0.03%, Allergan) which contains 0.005% BAK.8 These investigators found no significant difference among the three prostaglandins, either subjectively or objectively, after three months of treatment. Studies of longer duration are needed, but the work by Dr. Yamazaki was of the same length and had a very different outcome.
BAK and Glaucoma
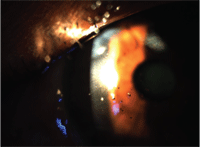
1. Patient with numerous metallic corneal foreign bodies.
A correlation between BAK-medications used in a glaucoma population was found to exist with lissamine green corneal and conjunctival staining.9 However, this study failed to show a relationship between utilization of BAK-containing medications with other dry eye evaluative testing (e.g., Schirmer tear tear or Tear Film Break-up Time [TBUT]). There was poor correlation between the Ocular Surface Disease Index questionnaire and the clinical measurements of ocular surface disease. Therefore, despite an increase in lissamine green staining when utilizing glaucoma medications with BAK, no conclusions can be made.
A group of 158 glaucoma patients experiencing ocular irritation secondary to latanoprost were switched to Taflotan (tafluprost, Santen Pharmaceuticals).10 Tafluprost is the only preservative-free (PF) prostaglandin and, although not available in the United States, it is found in some European countries. Patients’ intraocular pressure control was comparable when switched to the PF prostaglandin, but their tolerability was enhanced showing marked improvement in symptoms and signs when BAK was removed.
Choosing the Right Model
Some studies utilize a monolayer of transformed human corneal epithelial (HCE-T) cell cultures. But this cornea model may not adequately simulate the stratified nature of the cornea. This model has shown cytotoxicity to solutions found even to be non-toxic in rabbit corneas. Because of limitations with animal models and HCE-T, other models have been investigated. A 3D construct of immortalized human corneal epithelial cells—histologically similar to the human cornea—has been employed. Ocular irritancy test studies have been shown to have good reliability using this model.
In a study by Su Khoh-Reiter, and Bart Jessen, using this 3D construct model, cytotoxic effects of BAK-free travoprost were compared to latanoprost/BAK 0.02% and olopatadine/BAK 0.01%.11 They also instilled these drops into monkeys at a concentration far exceeding normal treatment levels for one year. No cytotoxicty was noted in either study for solutions containing BAK, compared to the other preservatives. Researchers concluded that BAK likely does not cause corneal toxicity when used at normal dosing schedules. Of course, the results of this study challenge the findings of other models.
Many of the studies have been performed using a rabbit model, preferred for ocular irritation studies due in part to the animal’s large cornea which aids in observation. However, when interpreting these studies, several differences should be considered. Rabbits have a nictitating membrane (third eyelid), which essentially increases the conjunctival surface area, likely resulting in a change in the pharmacokinetics of drug absorption. Rabbits also have a much larger conjunctival sac allowing larger quantities of drug to be retained. With thinner corneas, rabbits have reduced tear production compared to humans, which would result in increased drug concentration at the ocular surface.12 Rabbits also have a significantly lower blink rate—approximately three blinks per hour compared to the 800 per hour of humans—which translates to a slower removal of the drug.13 It is thought that the rabbit model is an oversensitive model, which has the advantage of increased safety but may also cause overly cautious management.
Although most data demonstrates deleterious effects of BAK on the ocular surface, there are some findings that are less conclusive. The ocular tolerability model utilized for the study should be given careful consideration before drawing conclusions that translate clinical practice.
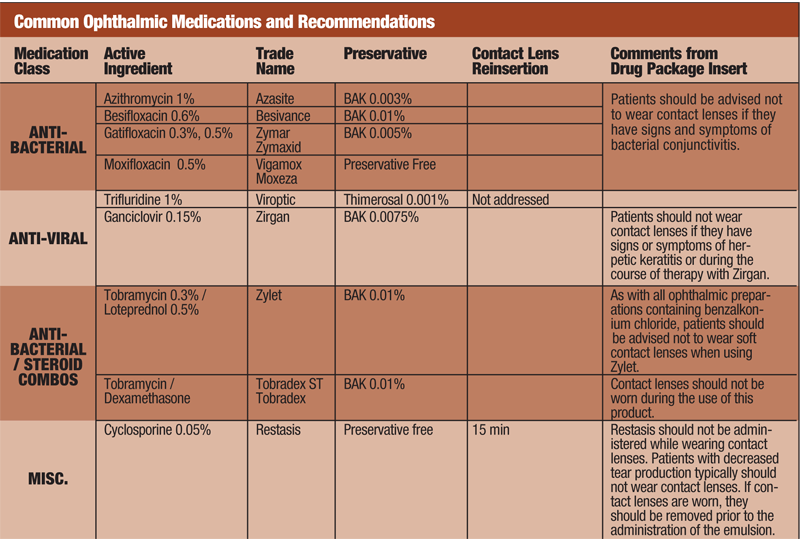
BAK and Contact Lenses
Much of the work in this area dates back at least a decade. Patients with early stages of giant papillary conjunctivitis are often eager to remain in their contact lenses while initiating topical therapy to quell their symptoms. Livostin (levocabastine 0.025%, CIBA Vision), which contains 0.015% BAK, was approved in 1993 as a topical ophthalmic antihistamine but has subsequently been discontinued. It has however been studied for use with contact lens wear.14 The study used rabbits, treated with one drop hourly for eight hours a day for six days. The absorption of active drug and BAK into soft contact lenses of differing water content levels was evaluated. Only a small amount of drug and BAK accumulated in the lenses, leading the authors to conclude that use of levocabastine (containing a relatively high concentration of BAK) for one week would not be problematic.14 Although this medication is no longer available, it can be concluded that other compounds containing BAK in similar amounts would likely be safe.
John Chapman, O.D., M.D., and colleagues also evaluated the interaction of BAK with contact lenses.15 Soft contact lenses of varying water content were soaked in radioactive C-14 BAK solution of 0.005% for seven continuous days. The uptake of BAK by the soft contact lenses was high with only a small percentage of washout in the first 24 hours. High-water contact lenses absorbed greater amounts of BAK than lower-water lenses. The authors concluded that the amounts of BAK retained and released by the contact lenses are of safety concern.15
Pierre Lumbroso, M.D., and colleagues expanded the list of preservatives evaluated; in addition to BAK, they also looked at thimerosal and chlorhexidine.16 The researchers found significant absorption of BAK and chlorhexidine by soft lenses. Thimerosal was weakly absorbed by high water soft lenses and no absorption was detected in lower water soft lenses.
Aphrodite Dracopoulos, M.Sc., and colleagues assessed the interactions of BAK with two silicone-containing contact lenses.17 They found a potentially irritating material was released after soaking these lenses even in BAK-free balanced salt solution. The authors speculate that the identity of this material may be a stabilizer, ultraviolet filter or a low molecular-weight additive. Lenses soaked in BAK-containing solutions caused damage to the corneal epithelium and mitochondrial metabolism.
Rigid gas permeable contact lenses typically do not absorb drugs. As pointed out by Joel Silbert, O.D., several factors of drug absorption into a soft contact lens need to be considered.18 Water content of the lens is important. High-water contact lenses will have a higher absorption rate of water-soluble compounds. The molecular weight of a drug is an important factor as well. Compounds with lower molecular weight (less than 500g/mol) can penetrate the contact lens matrix for later release. Lens thickness, drug concentration and the time that the lens is soaked in the drug are also factors. Dr. Silbert concluded that although not FDA-approved, it is probably acceptable to use topical medications containing BAK with a hydrogel lens as long as the lenses are not soaked in the products.18
Based on existing literature, the most common preservative—BAK—does compromise the cornea and is absorbed by contact lenses. Recently Masafumi Uematsu, M.D., and colleagues reported that by modifying the alkyl chain length on the BAK molecule, corneal toxicity could be reduced.19 So, perhaps a new and improved BAK is on the horizon!
Wait Time
In 1998, Mike Christensen, Ph.D., O.D., and colleagues conducted a creative study to address the question of how long patients should wait to reinsert their lenses after utilizing an ophthalmic drop.20 In this study, researchers used Naphcon-A (pheniramine maleate 0.3%/naphazoline hydrochloride 0.025%, Alcon) which contains 0.01% BAK. It was instilled with the contact lenses on the eye, instead of removing the lens five minutes before reinserting. In a healthy eye with an intact cornea with no contact lens wear, pupillary dilation does not typically occur. Therefore, if pupillary dilation occurred, it was attributed to increased absorption of the drug in the contact lens.
The researchers found that removing the contact lens for five minutes was no different than not wearing a lens at all. If the drop was instilled while wearing the contact lens, there was enough drug absorption to cause a physiologic effect (i.e., pupillary dilation). They also assayed the amount of BAK retained by the lenses. The BAK concentration contained within the contact lens that remained on the eye during drop instillation was statistically significantly higher than lenses that were off the eye for five minutes. They concluded that 10-minute lens removal was appropriate to prevent significant absorption.20 These findings confirmed previous reports, including a fluorophotometric evaluation, that found the majority of clearance of a drug occurs during the first five minutes.20
Improved Dosing
The most recently approved topical ophthalmic agents have one thing in common: reduced frequency of dosing. Examples include Moxeza (moxifloxacin 0.5%, Alcon) dosed twice a day, Bromday (bromfenac 0.09%, Ista Pharmaceuticals) dosed daily, Durezol (difluprednate, Sirion Therapeutics) typically dosed half as often as prednisolone acetate 1%, and Lastacaft (alcaftadine 0.25%, Vistakon) which also has a once a day dosing schedule. Less frequent dosing improves patient adherence and persistence, and reduces the exposure to preservatives.
In addition to finding vehicles that provide increased contact time with the eye, utilizing a contact lens as a delivery system has been investigated. There are currently several pending applications that utilize contact lenses as a drug depot to provide a sustained release of various pharmaceutical agents. The residence time of a drug in the eye—when applied in the presence of a contact lens—is 30 minutes, double the time when eye drops are applied without lens wear. There are several different ways that contact lenses can be utilized as the delivery system of drugs: soft contact lenses can be soaked in the desired drug; the drug can be incorporated into the solution prior to lens polymerization; or nanoparticles with the drug can be loaded into the lens gel matrix for extended delivery (see “The Future is Now: Unveiling Smart Contact Lenses”).21 In a recent study by Clyde Schultz, Ph.D., and colleagues, contact lenses were soaked in dilute solutions of timolol maleate or brimonidine and assayed for drug uptake and subsequent drug release.22 This study indicated that both of these drugs could be transferred to the contact lenses and then released over a period of 60 to 90 minutes. Volunteers then wore drug soaked lenses for 30 minutes. They concluded that this may be considered a viable method to control IOP with low dose of medication. Lens wear was well-tolerated and there were no adverse effects noted to the preservatives.
As researchers work to use contact lenses to deliver drugs, several challenges exist including the need to control the release of the drug and the ability to obtain a clinically significant concentration of the drug within the contact lens matrix. The answer might be in the field of molecular imprinting.23 Molecular imprinting within a gel involves macromolecular memory for a template molecule within a polymer network.
For the time being, the most prudent recommendation to follow is provided in the package insert for each drug regarding eye drops and contact lens wear. The most common recommendation is for lenses to be removed prior to drop instillation and then reinserted 10 to 15 minutes later. Commonly prescribed ophthalmic medications and the FDA recommended time before contact lens reinsertion are summarized.
The future of contact lenses as a delivery system is encouraging. Many of the drugs delivered will likely be preserved and, therefore, additional ocular tolerability studies will be needed. If and when sufficient evidence-based literature supports eye drop instillation while wearing contact lenses, our patients will benefit from convenience and likely better, consistent delivery of their medications.
Dr. Than is an associate professor at the University of Alabama at Birmingham School of Optometry where she teaches ocular pharmacology and anterior segment disease. She is also a clinical attending in the Ocular Disease Service at UAB Eye Care.
1. Abelson MB, Berdy GJ. The BAK story. Ophth Management. April 2009.
2. Kusano M, Uematsu M, Kumagami T, et al. Evaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivo. Cornea. 2010 Jan;29(1):80-5.
3. McCarey B, Edelhauser H. In vivo corneal epithelial permeability following treatment with prostaglandin analoges with or without benzalkonium chloride. J Ocul Pharmacol Ther. 2007 Oct;23(5):445-51.
4. Ammar DA, Noekcer RJ, Kahook MY. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv Ther. 2010 Nov;27(11):837-45.
5. Whitson JT, Cavanagh HD, Lakshman N, Petroll WM. Assessment of corneal epithelial integrity after acute exposure to ocular hypotensive agents preserved with and without benzalkonium chloride. Adv Ther. 2006 Sep-Oct;23(5):663-71.
6. Kahook MY, Noecker R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv Ther. 2008 Aug;25(8):743-51.
7. Yamazaki S, Nanno M, Kimura T, et al. Effects of switching to sofZia-preserved travoprost in patients who presented with superficial punctate keratopathy while under treatment with latanoprost. Jpn J Ophthalmol. 2010 Jan;54(1):7-14.
8. Whitson JT, Trattler WB, Matossian C, et al. Ocular surface tolerability in prostaglandin analogs in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2010 Jun;26(3):287-92.
9. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008 Aug;17(5):350-5.
10. Uusitalo H, Chen E, Pfeiffer N, et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2010 May;88(3):329-36.
11. Khoh-Reiter S, Jessen BA. Evaluation of the cytotoxic effects of ophthalmic solutions containing benzalkonium chloride on corneal epithelium using an organotypic 3-D model. BMC Ophthalmol. 2009 Jul;9:5.
12. Curren RD, Harbell JW. In vitro alternatives for ocular irritation. Environ Health Perspect. 1998 Apr;106 (Suppl 2):485-92.
13. Maurice D. The effect of the low blink rate in rabbits on topical drug penetration. J Ocul Pharmacol Ther. 1995 Fall;11(3):297-304.
14. Momose T, Ito N, Kanai A, Shibata M Adsorption of levocabastine eye drops by soft contact lenses and its effects in rabbit eyes. CLAO J. 1997 Apr;23(2):96-9.
15. Chapman JM, Cheeks L, Green K. Interactions of benzalkonium chloride with soft and hard contact lenses. Arch Ophthalmol. 1990 Feb;108(2):244-6.
16. Lumbroso P, Nhamias M, Nhamias S, Tranche P. A preliminary study of the adsorption and release of preservatives by contact lenses and collagen shields. CLAO J. 1996 Jan;22(1):61-3.
17. Dracopoulos A, Dixon D, Jones L, et al. In vitro assessment of medical device toxicity: interactions of benzalkonium chloride with silicone-containing and p-HEMA-containing hydrogel contact lens materials. Eye Contact Lens. 2007 Jan;33(1):26-37.
18. Silbert JA. A review of therapeutic agents and contact lens wear. J Am Optom Assoc. 1996 Mar;67(3):165-72.
19. Uematsu M, Kumagami T, Shimoda K, et al. Influence of alkyl chain length of benzalkonium chloride on acute corneal epithelial toxicity. Cornea. 2010 Nov;29(11):1296-1301.
20. Christensen MT, Barry JR, Turner FD. Five-minute removal of soft lenses prevents most absorption of a topical ophthalmic solution. CLAO J. 1998 Oct;24(4):227-31.
21. Kapoor Y, Thomas JC, Tan G, et al. Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials. 2009 Feb;30(5):867-78.
22. Schultz CL, Poling TR, Mint JO. A medical device/drug delivery system for treatment of glaucoma. Clin Exp Optom. 2009 Jul;92(4):343-8.
23. White CJ, Byrne ME. Molecularly imprinted therapeutic contact lenses. Expert Opin Drug Deliv. 2010 Jun;7(6):765-80.


