We are often pressed for time with our patients and, in an effort to quickly see one patient and move on to the next to increase patient volume due to low insurance reimbursements, may skip what seems like ancillary testing during contact lens evaluations. While maybe not a direct result, contact lens dropout rates remain high due to factors such as discomfort and vision-related problems.1 This makes it more important than ever to prioritize patient comfort and quality of care by taking the time to exercise basic contact lens exam principles and gather appropriate patient information upfront, thereby reducing contact lens problems down the road and increasing overall patient success and satisfaction.
We must first obtain thorough medical and ocular histories from our patients, including whether they wear or have worn contact lenses. ODs should consider taking anatomical measurements—horizontal visible iris diameter (HVID), pupil size in dim and bright illumination and palpebral aperture size—when necessary. We must also obtain a refraction, assess corneal curvature, evaluate binocular vision and perform a slit lamp evaluation. The following article discusses a few steps ODs should follow to carry out a successful contact lens fitting.
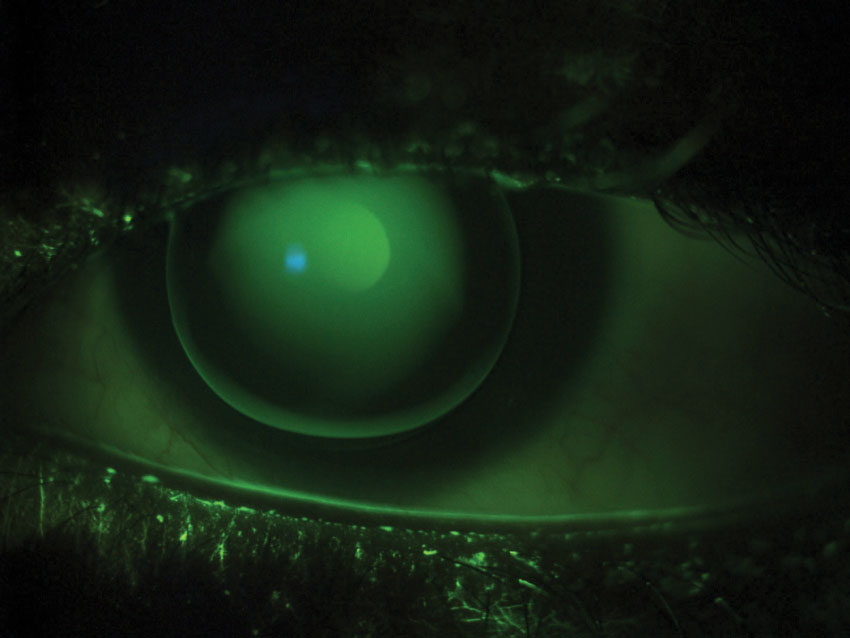 |
| Fig. 1. This patient has a high amount of astigmatism and wears a well-fitting GP. The fluorescein pattern shows a uniform green alignment and adequate edge lift. |
Measure the HVID
While soft lenses that are too small can cause irritation, soft lenses that are too large can cause insertion difficulties. Ordering the right contact lens parameters, therefore, is key and can be done by first taking a handful of anatomical measurements, including the HVID.
Ideally, a soft lens should overlap the limbal region by at least 0.5mm in every direction. As a general rule of thumb, adding 2.0mm to the HVID is a good approximation when determining an initial lens diameter that will comfortably fit an eye and provide enough coverage around the cornea.2
The average HVID is 11.8mm, so the majority of commercially available lenses range from 13.8mm to 14.5mm in diameter.3 If a patient falls outside of this range, a custom soft lens may be necessary, especially because lens coverage on the eye could already be less than expected. Decentration of the lens is one potential cause of this phenomenon. In addition, the HVID underestimates corneal diameter by approximately 1.0mm, and the limbal zone varies among patients.4 Soft lenses have also been shown to shrink several tenths of a millimeter when placed on the eye due to the change in temperature and pH.4 Taking this information into consideration when fitting contact lens patients can greatly improve your success rate.
 |
| Fig. 2. Here, we can see lissamine green staining of the conjunctiva in a patient with a history of a tight-fitting soft lens. |
Case Example #1
A 20-year-old Caucasian female presented for a contact lens evaluation. The patient reported poor comfort in her current toric daily disposable soft lenses, which had a base curve (BC) of 8.5mm and a diameter of 14.5mm. Her vision with the current lenses was 20/20 OU, and ocular health findings were unremarkable. Upon evaluating the patient’s current lens fit, we found adequate lens movement but temporal decentration and poor coverage over the nasal limbus OU.
Manifest refraction revealed:
- +0.50 -1.25x170 (VA of 20/20) OD
- -0.50 -1.25x180 (VA of 20/20) OS
The patient’s keratometry readings were 43.00/43.75@085 OD and 42.75/44.25@085 OS with clear and regular mires OU. Her HVID was larger than average at 13.1mm. We ordered custom C-vue soft toric lenses with a BC of 8.7mm and a diameter of 15.0mm. The patient maintained 20/20 vision OU with the new lenses, which had better centration and coverage over the limbal region, and reported an improvement in comfort compared with her previous lenses.
Find the Corneal Curvature
Corneal curvature should be evaluated in every contact lens patient. This is often done by keratometry or autokeratometry, which only measures the central 3.0mm of the cornea. Corneal topography provides more detailed information on corneal shape and usually measures out to 9.0mm to 10.0mm. Regardless, evaluating corneal curvature helps ODs determine which lens has the best chance of successfully fitting certain patients.
Patients with small amounts (<1.00D) of corneal astigmatism can often be managed successfully in a soft lens. Patients with moderate amounts (1.00D to 3.00D) may benefit from a spherical gas permeable (GP) lens, while patients with high amounts (>3.00D) may benefit from a bitoric GP.5
According to a survey of diplomates in the Cornea and Contact Lens Section of the American Academy of Optometry, the majority of ODs prefer using bitoric GPs at 2.00D to 2.75D of corneal cylinder and implementing the Mandell-Moore guide when fitting these lenses.6 VA measurements do not differ significantly between soft lenses and GPs, and patients with moderate to severe amounts of astigmatism often report an improvement in the quality of their vision with GP lenses.7
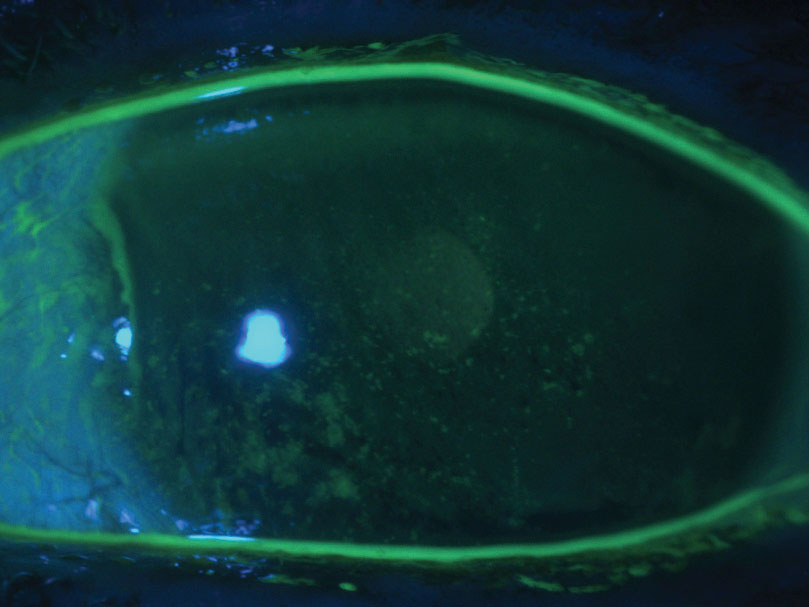 |
| Fig. 3. This patient has severe dry eye and diffuse punctate epithelial defects. |
Case Example #2
A 27-year-old Asian male was referred by his MD for a contact lens evaluation. He had a history of soft lens use but reported discontinuing lens wear because he achieved better vision with glasses.
Manifest refraction revealed:
- -6.50 -4.50x005 (VA of 20/20) OD
- -8.50 -3.50x170 (VA of 20/20-) OS
We obtained Pentacam topography images, which revealed average pachymetry values, and did not find any abnormalities on the posterior or anterior elevation maps of either eye. We did find a large amount of corneal cylinder—3.30D OD and 3.10D OS—that led to us ordering bitoric GP lenses by X-Cel for the patient.
The amount of corneal and refractive astigmatism were not equivalent in the patient’s right eye, so we ordered a lens with a cylinder power effect design with a diameter of 9.3mm, a power of -6.00D/-9.00D and a BC of 44.25D/46.75D. His left eye, on the other hand, had a similar amount of corneal astigmatism and refractive astigmatism, so we ordered a lens with a spherical power effect design with a diameter of 9.3mm, a power of -7.75D/-9.75D and a BC of 45.00D/47.25D. This type of bitoric lens has the added benefit of rotating without inducing any astigmatism or causing blurry vision.
We achieved an adequate alignment fit with the new lenses upon observation with fluorescein, and the patient achieved 20/20 vision OU (Figure 1). He also reported an improvement in comfort.
Evaluate the Ocular Surface
Carefully checking the ocular surface for irregularities is an important part of any contact lens evaluation, as approximately 30% to 50% of contact lens wearers report dry eye (DE) symptoms.8
During slit lamp examinations, lids and lashes should be carefully evaluated for any signs of blepharitis or meibomian gland dysfunction; contact lens wear has been associated with meibomian gland dropout, lower quality meibum and increased lid margin abnormalities.8 The conjunctiva should be evaluated by eyelid eversion, and conjunctival staining with lissamine green should be used over rose bengal, as it does not sting as much and has been helpful in diagnosing DE (Figure 2).9 The tear film and cornea should also be carefully evaluated with fluorescein staining. Based on the severity of the case, additional DE testing, such as osmolarity or MMP-9 testing, may be necessary.
For patients with moderate to severe ocular surface disease (OSD) who were not successful with more conservative treatment options, such as drops or punctal plugs, scleral lenses may be the solution. Scleral lenses can heal the cornea and resolve epithelial defects by providing mechanical protection and continuous hydration. Studies have demonstrated the safety and efficacy of scleral lenses in improving ocular comfort, protecting the ocular surface and resolving epitheliopathy in patients with various types of OSD, including neurotrophic keratopathy, exposure keratopathy and limbal stem cell deficiency.10 In any case, DE should be managed appropriately prior to initiating contact lens wear.
 |
| Fig. 4. This optic section shows a 1:1 ratio of lens thickness to tear film layer in a scleral fit. We are comparing the dark layer (lens thickness) to the tear film layer (green band). |
Case Example #3
A 50-year-old female with a history of severe DE was referred for a corneal evaluation. The patient had been prescribed various artificial tears, ointments and steroids but experienced minimal relief. She was motivated to resume contact lens wear but reported poor vision in previous monovision soft lenses and custom soft toric multifocals. A slit lamp evaluation showed diffuse punctate epithelial erosions OU (Figure 3).
Manifest refraction revealed:
- -11.00 -1.00x035 (VA of 20/40) OD
- -10.75 -0.50x115 (VA of 20/40) OS
We managed the patient’s DE by prescribing preservative-free tears and ointment for use at night. Unfortunately, the patient deferred treatment with topical cyclosporine and an amniotic membrane due to cost concerns. More consistent lubrication improved her ocular surface, and we fit her in scleral multifocals by Valley Contax due to ocular surface concerns and vision demands. The scleral fit showed adequate vault and edge alignment, and the patient achieved 20/20 vision OU (Figure 4). After several weeks of wearing the lenses, the patient’s ocular surface showed improvement.
You Get Out What You Put in
The most important lesson for ODs who fit patients with contact lenses is to remember basic contact lens principles and gather appropriate information during initial contact lens evaluations no matter how much time it takes and how many patients you have to see. Putting in the time and effort upfront pays off in the long run and leads to more successful, satisfied patients.
Dr. Frantzis is an assistant clinical professor at the SUNY College of Optometry. She specializes in complicated contact lens fittings and myopia control.
Dr. Duchnowski is chief of the Contact Lens Service at the University Eye Care Center and director of the Cornea and Contact Lens externship at the SUNY College of Optometry.
1 . Sulley A, Young G, Hunt C. Factors in the success of new contact lens wearers. Cont Lens Anterior Eye. 2017;40(1):15-24. 2. Bennett ES, Henry VA. Clinical manual of contact lenses. 4th edition. 2014. 3. Caroline PJ, André MP. The effect of corneal diameter on soft lens fitting, part 1. Cont Lens Spectrum. 2002;17(4):56. 4. Jones L, Brennan NA, González-Méijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):13-13215. 5. Kastl PR. Correction of astigmatism with rigid gas permeable lenses. Ophthalmol Clin North Am. 2003;16(3):359-63. 6. Blackmore K, Bachand N, Bennett ES, et al. Gas permeable toric use and applications: survey of Section on Cornea and Contact Lens Diplomats of the American Academy of Optometry. Optometry. 2006;77:17-22. 7. Michaud L, Barriault C, Dionne A, et al. Empirical fitting of soft or rigid gas permeable contact lenses for the correction of moderate to severe refractive astigmatism: a comparative study. Optometry. 2009;80(7):375-83. 8. Arita R, Fukuoka S, Morishige N. Meibomian gland dysfunction and contact lens discomfort. Eye Cont Lens. 2017;43(1):17-22. 9. Perry HD, Donnenfeld ED. Dry eye diagnosis and management in 2004. Curr Opin Ophthalmol. 2004;15:229-304. 10. Schornack MM, Pyle J, Patel SV. Scleral lenses in the management of ocular surface disease. Ophthalmology. 2014;121(7):1398-1405. |


