 |
Consumers trust the FDA to regulate products and keep us safe, and to address any product-related issues should they arise. Following the 2006 Fusarium keratitis outbreak and the 2007 Acanthamoeba cases, which resulted in voluntary recalls of two contact lens solutions, the Center for Disease Control (CDC) and the FDA initiated investigations into the root cause of these serious ocular infections. Their findings led the FDA to reevaluate solution testing in the following areas:
(1) Lens compatibility—does the solution alter the parameters of the lens?
(2) Cleaning efficacy, determined through the use of Critical Micelle Concentration (CMC).
(3) Microbial testing, with the use of predetermined microbes.
(4) Toxicology testing, to determine cytotoxicity, systemic toxicity and ocular irritation.
(5) Clinical testing, with a minimum of 60 subjects over a period of one month.
In the June 2014 issue, we examined lens compatibility, in particular how materials affect the uptake and release patterns of solution components, and September 2014 we discussed the efficacy of the contact lens cleaning process. This month, we will focus on microbial testing.
Aye, There’s the Rub
Historically, the FDA used two different methods—“rub” and “no rub”—to evaluate microbial efficacy for contact lens solutions. In the “no rub” method, a known cultured organism is exposed to a preset quantity of contact lens solution. If the organism dies as a result of this process, the solution would receive FDA approval for disinfection and could be labeled “no rub,” meaning that no rubbing motion is required for the solution to be effective. If a significant amount of organism remains during the “no rub” test, however, then the solution would require a “rub” method.
In the “rub” method, a lens is exposed to an organism, rubbed and rinsed for five seconds and then cultured for growth. A solution that passes the criteria for this method but does not pass the “no rub” conditions requires the rub and rinse step to be labeled on the packaging.
| |
|
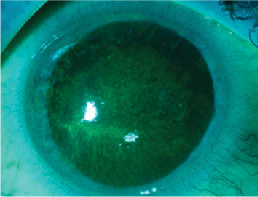
Dense epitheliopathy and a pseudodendrite on initital presentation of Acanthamoeba infection.
|
Unfortunately, neither of these methods took “real-world” factors into account, such as Acanthamoeba testing, uptake and release of preservatives, common impurities both in the lens case and on the lens, the effect of the lens case on the solution’s composition and patient-related behaviors such as topping off of pre-existing contact lens solution in the case. Additionally, it was not considered that microbe strains repeatedly grown on cultures might lose virulence and affect testing outcomes.
The FDA has now proposed adding Acanthamoeba to its testing regimen, accounting for at least two different strains and increasing the challenge level of the free-living amoeba’s transformation into a more durable cyst. Additionally, it will focus on the importance of lens/solution interactions, specifically the depletion of the solution biocide (uptake and release) in the presence of a lens, as well as real-world non-compliance issues.
The FDA has also removed the “no-rub” label from all packaging and has increased its public awareness campaign on healthy contact lens wear.1
Manufacturers must constantly balance the ability of a solution to kill organisms with potential toxicity to the ocular surface. In the next column, we will discuss the role of toxicology in lens care products.
1. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ContactLenses/default.htm


