Current scleral lenses present a remarkable modern option for refractive correction and ocular protection in a variety of distinct ocular indications. Firstly, they provide marked improvements in comfort and visual stability for individuals with irregular corneal astigmatism, the most common indication for scleral lenses (SLs) in the United States.1-5 Additionally, SLs are increasingly being used to treat dry eye diseases as well as patients with “normal” corneas.6-12 The decision to use a scleral lens begins with logical and basic decision-making to weigh benefits of using the device, and while specialists are certainly managing complex scleral lens fits, basic management can be practiced by any motivated eyecare practitioner. Here we will discuss the basic fitting considerations and techniques for entry-level management of patients with scleral lenses.
 |
|
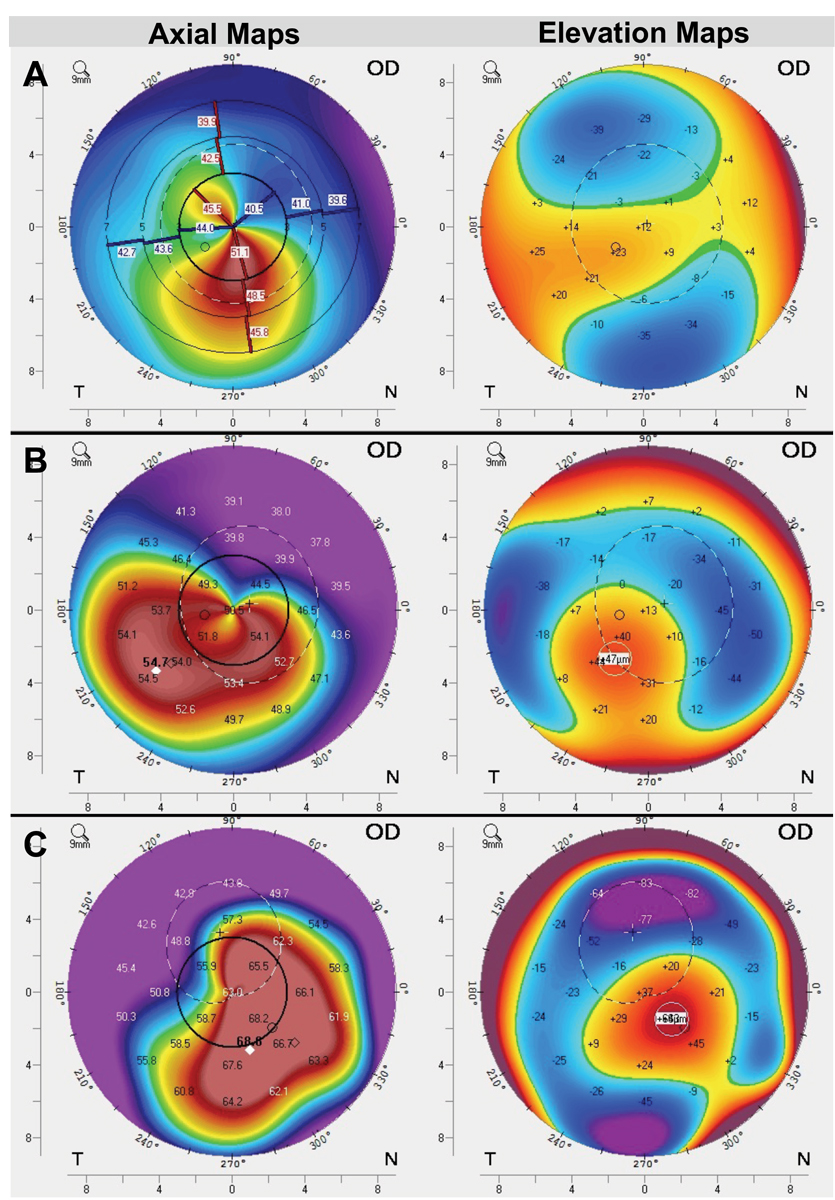
Fig. 1. Assess corneal elevation maps for scleral lens suitability. Here, corneal topography from three patients with irregular astigmatism shows one patient (A) with relatively mild elevation differences (~60um) and a relatively symmetric shape. Another patient (B) shows moderate elevation differences (~95um) as well as greater asymmetry, and a third patient (C) with severe elevation differences (~150um) as well as high amounts of asymmetry. Our rule-of-thumb is that if there is greater than about 90um of elevation differences between peak and trough on the cornea, scleral lenses should automatically be the priority choice. While all of these patients could be good candidates, patient B and C are particularly indicated due to high asymmetry and elevation differences on the anterior cornea. Click image to enlarge. |
The Right Option
Scleral lenses are becoming readily available worldwide, and in the US alone there are over 20 manufacturers. Each of them has designed practitioner-friendly fitting sets and offer simple as well as sophisticated lens customizations to manage a variety of ocular shapes and conditions. This and the ease of obtaining high quality scleral lens education from free resources like the Scleral Lens Education Society (SLS) have made the accessibility of lens fitting expand to many practitioners over the past decade; the beginner need not be intimidated.
The first decision a practitioner must make is whether a patient can benefit from a scleral lens. This may seem obvious, but a common mistake is to think that all patients will have a perceived benefit from these devices. Critically weighing the benefits against the potential risks or downfalls of scleral wear before beginning the fitting process will save practitioners and patients time and energy. Scleral lens wearers typically come from one of three categories: the irregular cornea, ocular surface diseased and miscellaneous ‘normal’ category that encompasses high ametropia, presbyopia and other relatively normal conditions.
Irregular cornea. The most common SL indication in most practices is the irregular cornea, such as keratoconus and other ectasias.4,5 Corneas that are highly irregular in shape (i.e., have high differentials between the elevations and depressions on the anterior corneal surface) present a major challenge when fitting relatively small diameter corneal gas permeable (GP) lens, which have been the standard of care since the mid-20th century (Figure 1). Corneal GP lenses are supported by the shape of the cornea and often become unstable as disease severity increases.13 Scleral lenses vault over the irregular cornea, landing on the conjunctiva overlying the sclera and largely avoiding the effects of an unstable lens.
Patients with lesser amounts of corneal irregularity often still prefer a scleral lens, which is surpassing the corneal GP lens as standard of care due to the improved visual stability and ocular comfort. Additional considerations when deciding if a scleral is the right choice for irregular corneas, as well as with other indications, are palpebral aperture size (larger is favorable, although not necessary, for lens wear), tolerability (e.g., comfort), handling capacity (e.g., ability to apply/remove), and overall motivation to manage these lenses that undoubtedly require more investment than other lens types.
In addition to corneal ectasias, individuals who have undergone corneal surgical procedures, such as radial keratotomy, LASIK or keratoplasties, who also often have irregular corneas, are likewise good potential candidates for scleral lens wear.
 |
|
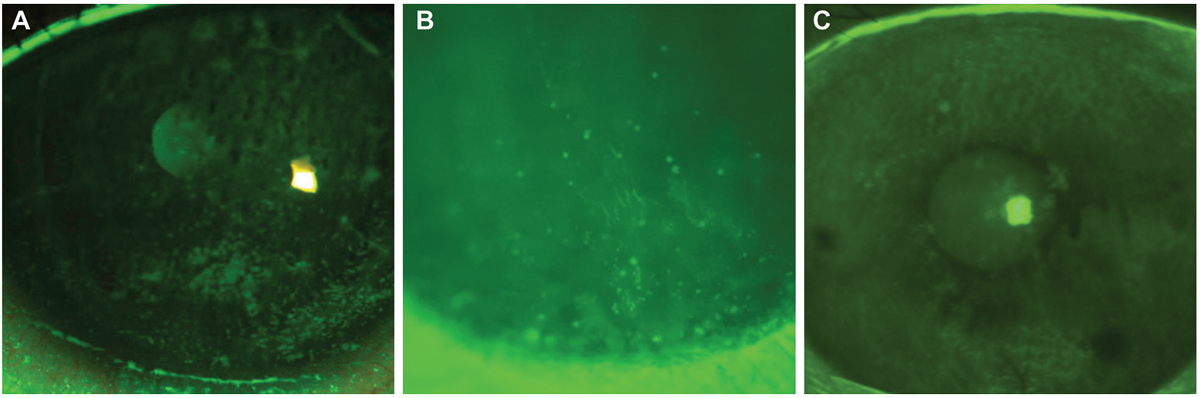
Fig. 2. In corneal staining post-lens removal, punctate staining is often observed after scleral removal, seen in a post-transplant patient (A) and to a lesser extend in a keratoconus patient (B). Epithelial bogging (C) is also a common finding that does not appear to have any adverse effect on the cornea but is not well understood. Each example was within the normal acceptable range for these patients but underlies the importance of baseline testing to monitor changes in staining. Click image to enlarge. |
Ocular surface disease. Incorporating scleral lenses into an ocular surface disease (OSD) management strategy is usually more complicated than fitting irregular corneas. Although the fitting process is often straightforward when there is a normal ocular (i.e., corneal) shape, managing OSD can be challenging. Scleral lenses are often not considered an initial therapy option for classic dry eye diseases, as many practitioners prefer more conservative approaches first, such as soft bandage lenses, topical lubricants or steroids, cyclosporine and punctal occlusion.14 The DEWS II report agrees and recommends implementing scleral lenses after these approaches, likely due to the lack of evidence that these lenses are appropriate for the majority of patients in the mild to moderate classes of dry eye disease.15
For practitioners beginning with scleral lenses, we recommend approaching “dry eye” lens fits with caution due to their mixed efficacy. However, in severe disease, these lenses are essential and are often combined with the aforementioned therapies as well as autologous serum tears or amniotic membrane grafting in patients such as those with severe systemic pathologies including Sjögren’s syndrome, ocular cicatricial pemphigoid, Stevens-Johnson syndrome or graft-vs.-host disease. These diseases often require comanagement with subspecialists including ophthalmology, rheumatology or oncology, and are out of the scope of this discussion of basic scleral lens management.
“Normal” cornea. Many practitioners are reporting fitting sclerals for “normal eye” indications, such as high ametropia, presbyopia and non-pathologic dry eye. While there are certainly patients who benefit from these lenses, there are no available studies on success rates of these lenses with normal patients, and scleral lenses are likely to be less comfortable than soft lenses in the normal population.16 The normal patient who is interested in these lenses should be scrutinized to determine that the benefit (i.e., vision, comfort) is greater than the risks, costs and inconveniences that sclerals can bring. For a beginning scleral lens fitter, we recommend that this group should be avoided until the practitioner feels comfortable with their understanding of vision and comfort improvements that the lenses provide.
A Prior Evaluation
Once a scleral is predicted to be the best option for a patient, evaluate and carefully document a baseline level of anterior segment and ocular surface disease. Corneal topography and a slit lamp to assess the ocular surface, including eyelid margin and palpebral conjunctival assessment, are all that are necessary for basic baseline testing. Documentation should include staining patterns, scarring, tissue thinning/thickening and all other normal and abnormal findings.
Many features of the ocular surface, such as tear parameters, staining and corneal curvature and thickness, have been shown to be altered post-scleral lens wear and thus should be carefully assessed prior to wear (Figure 2).17-23 Especially when managing dry eye, features like tear volume, osmolarity, tear break up time, as well as symptom surveys (e.g., OSDI, SPEED) can be helpful in the baseline assessment for lens suitability as well as in monitoring changes with scleral lenses.
Fitting a Lens
Let’s consider the multiple-step process and different methods to fit a scleral on a patient.
Diagnostic fitting. This method has been streamlined with easy-to-use lens fitting sets and detailed guides providing step-by-step instructions. Start by choosing an appropriate lens diameter; this is often dependent on the horizontal visible iris diameter, palpebral aperture size and severity of disease. Larger corneal diameters with more severe disease may benefit from a larger lens (i.e., >16mm), whereas patients with small palpebral apertures may require a smaller lens for ease of application. Note that these are common guidelines, but many practitioners develop a preference for one or two diameters and only use smaller or larger lenses for special cases. After selecting the lens diameter, the fitting guide should direct to a starting sagittal depth and or lens shape (i.e., prolate vs. oblate) depending on the patient characteristics.
Slit lamp assessment and (optional) OCT. Once the diagnostic lens has been selected, clean and condition it well and apply to the eye using preservative-free saline and add sodium fluorescein to visualize the fluid reservoir. Confirm that no application bubbles are present, the lens is wetting properly and that the cornea appears adequately vaulted by viewing the eye in low mag, full illumination cobalt blue light (Figure 3).
After that initial assessment, the amount of lens vault and the apposition of the landing zone can be more specifically evaluated. A high illumination, white light optic section is the best at determining corneal vault (Figure 4). Aim for approximately 250µm to 300µm of initial vault pre-lens settling, as scleral lenses will settle into the conjunctiva and vault will be reduced. While the amount of settling is variable among different lens designs and eyes, practitioners should anticipate at least 75µm to 100um settling, about 50% of which will likely occur within the first hour.24-28
To assess the landing zone, low magnification, dim white-light is the best technique to evaluate vessel blanching, edge-lift and other subtle landing zone findings. The scleral lens should land on the conjunctiva outside of the limbal margin and should have minimal impingement of conjunctival blood vessels. Many patients will need a toric- or quadrant-specific landing zone to accommodate toric or asymmetric scleral curvatures; therefore, fitting sets often include lenses with toric landing zones, which can be useful to determine how a toric lens will rotate on the eye and where the relatively flat and steep meridians lie.29-31
Areas of uncertainty when assessing the scleral lens fit using the slit lamp can be more precisely evaluated using anterior segment optical coherence tomography (AS-OCT), which provides a high-resolution view of the lens-to-cornea relationship in primary and extreme gazes. This technique can be helpful in providing exact measurements of corneal vault and revealing subtle edge misalignments but is not essential for a beginning scleral lens practitioner.
 |
|
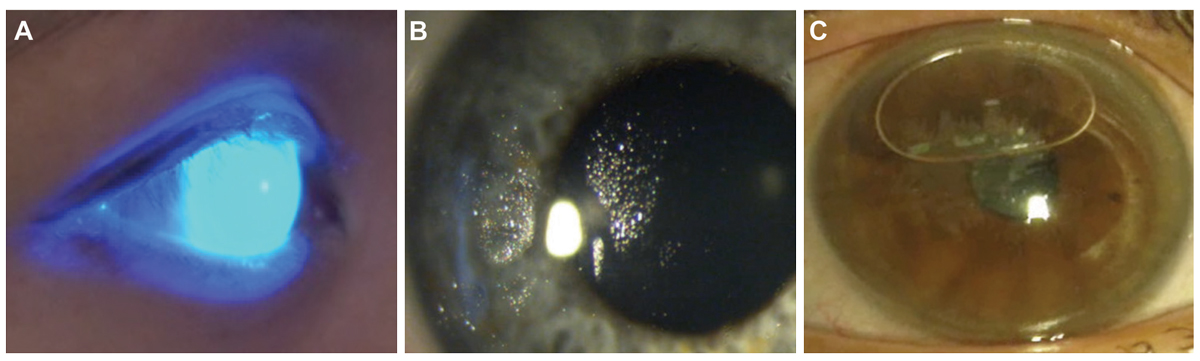
Fig. 3. When using sodium fluorescein for an initial assessment of a scleral lens on the eye, a widefield, low mag view of the lens in cobalt blue light (A) will allow a first view of the lens fit, which appears adequate here with no areas of darkness that would indicate touch or a bubble. This can be observed using a slit lamp or using a handheld light source. Non-wetting lenses (B) and application bubbles (C) are common and can often be viewed outside of the slit lamp. Both indicate that a lens should be removed, reconditioned if needed, and reapplied carefully. Click image to enlarge. |
Empirical fitting. The advent of scleral topography (i.e., profilometry) has paved the way for empirical scleral fitting and is especially beneficial when fitting an eye with prominent conjunctival elevations or rotational asymmetry.30,32
Scleral shape data drives the lens customization process and is directly transmitted to manufacturers who can design more precise toric or quadrant-specific curvatures and even more advanced freeform lens designs.29,31,33,34 When using this type of fitting system, an initial profilometry scan is taken and a diagnostic lens is applied to determine the over-refraction. This data is sent to the lab where the lens shape is designed using software and power is determined based on the over-refraction.
Empirical scleral lens fitting can reduce chair time and may become the predominant method of choice as scleral profilometry becomes more readily available. While this could be quite useful for beginners in lens design, is not essential and should not be considered necessary for a basic scleral fitter.
Refractive considerations. Determining the scleral lens power during the fit is similar to that done with other diagnostic lenses. Although empirical power determination is possible with normal corneal shapes, the calculations are not reliable for irregular corneas. Best practice is to apply a diagnostic lens to the eye to determine the lens power. Spherocylindrical over-refraction should be done, but we recommend starting with the spherical equivalent power in the first lens order unless the residual astigmatism is convincing (e.g., >0.75D with a strong visual improvement). When in doubt, order a spherical equivalent first, and front surface toricity can be added to the lens after post-settling lens rotation is established.
Although it is possible, our experience is that flexure is rare with scleral lenses in the currently available materials and standard lens thicknesses. More commonly, lens decentration, which will typically be greatest when there is high nasal-temporal scleral asymmetry or excessive lens vault, can induce aberrations that will be reduced if better centration can be achieved (i.e., using toric, quadrant-specific, or other advanced landing zone technology).6
Tips for Initial Fitting:
- Apply a scleral lens to the eye early in the decision-making process to gauge patient tolerance and ease of fit.
- Use proparacaine if there is any difficulty with applying the lens.
- To get a dry diagnostic lens to wet properly on the eye, best practice is to clean well with a (sudsy) surfactant cleaner followed by a short soak in conditioner.
- When first starting with scleral lenses, if there is no scleral topography available, avoid using quadrant specific landing zone designs unless there is a very obvious need for one. Toric landing zones are much easier to troubleshoot and manage.
- Perform retinoscopy over the lens to determine the best starting point for over-refraction, which will improve refractive efficiency and allow a deeper understanding of the amount of irregularity that the lens is truly masking (based on the quality of the reflex).
Dispensing and Management
Scleral lenses are customarily ordered at the completion of the fitting visit and the customized lens is dispensed at a follow-up visit scheduled one to two weeks later. This period is a good time to direct the patient toward materials to learn about wearing SL and the process of application and removal. The SLS has several fitting videos and resources for patients (www.sclerallens.org). Additionally, patients can be directed to social media platforms, blogs and other online resources that can be helpful to connect them with peers and prepare them for wearing SLs.
The purpose of the dispensing visit is to confirm that the lenses are a good starting point, teach application and removal and hopefully dispense the scleral lens to the patient. In addition, a specific plan for lens hygiene should be developed. While many practitioners develop preferences for certain disinfection, conditioning and application solutions, is it good to remember that at least some personalization for different patient attributes must be considered.
We recommend starting with a peroxide or one-step multipurpose cleaner for disinfection and conditioning, along with an available preservative-free application solution. The lens care routine should be re-evaluated and changed as needed at each follow-up visit to determine to best plan for each patient.
 |
|
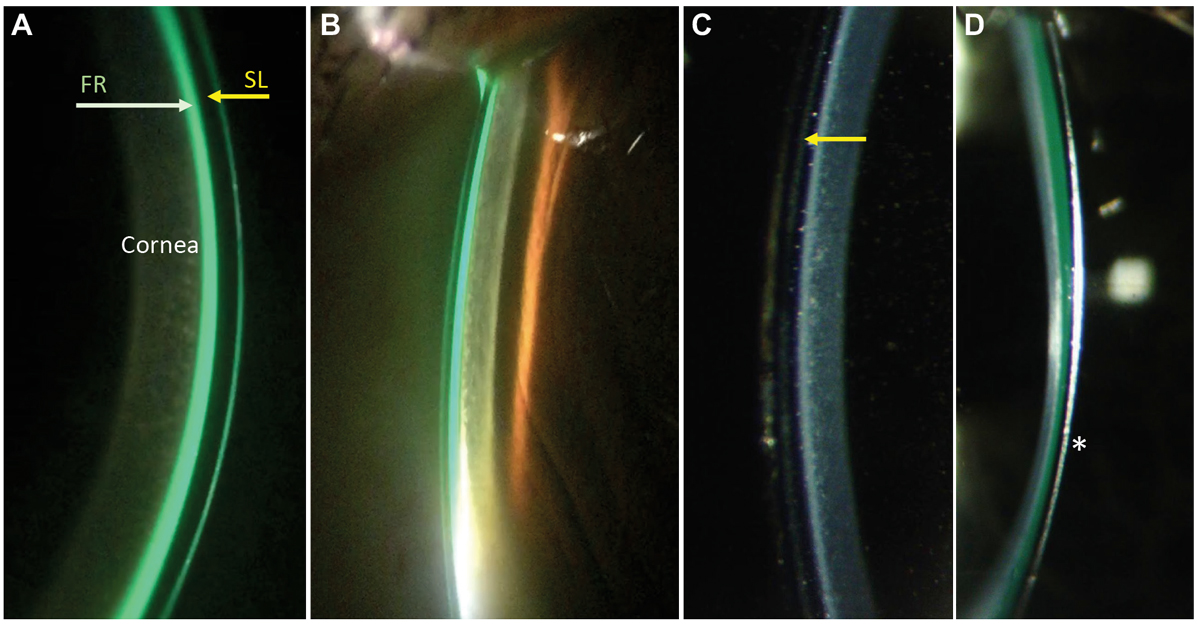
Fig. 4. To evaluate corneal vault in scleral lenses, measure the vault of a lens using a high illumination, medium/high magnification, optic section white light. Starting with the central cornea, estimate the lens vault by comparing the thickness of the lens to the fluid reservoir, labeled in (A) showing that the vault is approximately 150um superiorly (i.e., half the lens thickness), and slightly greater, approximately 200um, inferiorly. The optic section can be moved out toward the limbus (B) to likewise estimate the corneal vault. As an observer becomes more experienced they will gain proficiency in assessing the vault without sodium fluorescein in the fluid reservoir (C), where the back surface of the lens (arrow) can be detected best with the same slit lamp optic section technique. In highly irregular corneas such as keratoconus (D), the asymmetry of the vault may be more apparent with the lowest vault usually occurring at the apex of the ectasia (star). Click image to enlarge. |
Follow-Up
The follow-up schedule for scleral patients is somewhat specific to the disease being managed. During the initial fitting process, a patient should be monitored one to two weeks after initial dispense, sooner for patients who are high-risk or who are having difficulties with the application or removal process, and then again a few weeks after the final lens has been dispensed for a final check. It is not uncommon to need several additional visits between to modify lens power and parameters; most lens manufacturers have at least a 90-day warranty in which three or more lenses can be made with modifications until the final lens is determined.
Once established, SL wearers can often be monitored yearly, although some should be monitored at bi-annual or more frequent intervals to manage their underlying disease. Scleral lenses should be replaced every one to two years, although in some cases they can last longer if maintained well.
Testing at the follow-up visits are also somewhat disease-specific but should be considered for all scleral wearers. Patients should always be asked to apply their scleral lenses at least four hours before coming in for all follow-up visits, and the ocular surface should be assessed for staining and compared with the baseline.
In initial wearers, we recommend re-measuring corneal topography and intraocular pressure immediately after removing lenses, since these sensitive outcomes can be affected.35-37 Refer to the many resources on management of scleral lens complications for a more thorough description of the problems that can occur with sclerals and how to manage them.
Scleral lens fitting is rewarding for both patients who wear them and the eye care practitioners who manage them. Through this guide on basic scleral lens management, we hope to help novice practitioners gain confidence with scleral lens management and bring these remarkable devices to patients in their community that can benefit from their use.
Dr. Walker and Dr. Lee are assistant professors at the University of Houston College of Optometry. Dr. Walker’s PhD work centered on evaluating the inflammatory response of the eye to scleral lenses. The two are fellows of the American Academy of Optometery and the Scleral Lens Education Society.
1. Pullum KW, Buckley RJ. A study of 530 patients referred for rigid gas permeable scleral contact lens assessment. Cornea. 1997;16(6):612-22. 2. Yan P, Kapasi M, Conlon R, et al. Patient comfort and visual outcomes of mini-scleral contact lenses. Can J Ophthalmol. 2017;52(1):69-73. 3. Bergmanson JP, Walker MK, Johnson LA. Assessing scleral contact lens satisfaction in a keratoconus population. Optom Vis Sci. 2016;93(8):855-60. 4. Nau CB, Harthan J, Shorter E, et al. Demographic characteristics and prescribing patterns of scleral lens fitters: the scope study. Eye Contact Lens. 2018;44:S265-72. 5. Fuller D, Wang Y. Safety and efficacy of scleral lenses for keratoconus. Optom Vis Sci. 2020;97(9):741-8. 6. Schornack MM, Pyle J, Patel SV. Scleral lenses in the management of ocular surface disease. Ophthalmology. 2014;121(7):1398-1405. 7. Tappin MJ, Pullum KW, Buckley RJ. Scleral contact lenses for overnight wear in the management of ocular surface disorders. Eye (Lond). 2001;15(Pt 2):168-72. 8. Kok JH, Visser R. Treatment of ocular surface disorders and dry eyes with high gas-permeable scleral lenses. Cornea. 1992;11(6):518-22. 9. Romero-Rangel T, Stavrou P, Cotter J, et al. Gas-permeable scleral contact lens therapy in ocular surface disease. Am J Ophthalmol. 2000;130(1):25-32. 10. Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31(3):130-4. 11. Rosenthal P, Cotter J. The Boston scleral lens in the management of severe ocular surface disease. Ophthalmol Clin North Am. 2003;16(1):89-93. 12. Barnett M, Ross J, Durbin-Johnson B. Preliminary clinical exploration of scleral lens performance on normal eyes. J Contact Lens Res Sci. 2018;2(2):e14-21. 13. Lim L, Wen E, Lim L. Current perspectives in the management of keratoconus with contact lenses. Eye (Lond). 2020;34(12):2175-96. 14. Harthan J, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom (Auckl). 2018;10:65-74. 15. Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15(3):575-628. 16. Walker M. The Impact of a Scleral Lens on the Eye. PhD Dissertation. University of Houston; 2021. Accessed September 1, 2022. 17. Carracedo G, Serramito-Blanco M, Martin-Gil 19. Nixon AD, Barr JT, VanNasdale DA. Corneal epithelial bullae after short-term wear of small diameter scleral lenses. Cont Lens Anterior Eye. 2017;40(2):116-26. 20. Severinsky B, Behrman S, Frucht-Pery J, Solomon A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty: long-term outcomes. Cont Lens Anterior Eye. 2014;37(3):196-202. 21. Soeters N, Visser ES, Imhof SM, Tahzib NG. Scleral lens influence on corneal curvature and pachymetry in keratoconus patients. Cont Lens Anterior Eye. 2015;38(4):294-7. 22. Kumar M, Shetty R, Lalgudi VG, Vincent SJ. Scleral lens wear following penetrating keratoplasty: changes in corneal curvature and optics. Ophthalmic Physiol Opt. 2020;40(4):502-9. 23. Serramito-Blanco M, Carpena-Torres C, Carballo J, et al. Anterior corneal curvature and aberration changes after scleral lens wear in keratoconus patients with and without ring segments. Eye Contact Lens. 2019;45(2):141-8. 24. Esen F, Toker E. Influence of apical clearance on mini-scleral lens settling, clinical performance and corneal thickness changes. Eye Contact Lens. 2017;43(4):230-5. 25. Kauffman MJ, Gilmartin CA, Bennett ES, Bassi CJ. A comparison of the short-term settling of three scleral lens designs. Optom Vis Sci. 2014;91(12):1462-6. 26. Otchere H, Jones LW, Sorbara L. Effect of time on scleral lens settling and change in corneal clearance. Optom Vis Sci. 2017;94(9):908-13. 27. Bray C, Britton S, Yeung D, et al. Change in over-refraction after scleral lens settling on average corneas. Ophthalmic Physiol Opt. 2017;37(4):467-72. 28. Courey C, Michaud L. Variation of clearance considering viscosity of the solution used in the reservoir and following scleral lens wear over time. Cont Lens Anterior Eye. 2017;40(4):260-6. 29. Jesus DA, Kedzia R, Iskander DR. Precise measurement of scleral radius using anterior eye profilometry. Cont Lens Anterior Eye. 2017;40(1):47-52. 30. Visser ES, Visser R, van Lier HJJ. Advantages of toric scleral lenses. Optom Vis Sci. 2006;83(4):233-6. 31. Denaeyer G, Sanders DR, van der Worp E, et al. Qualitative assessment of scleral shape patterns using a new widefield ocular surface elevation topographer: the SSSG Study. JCLRS. 2017;1(Group 4):12-22. 32. Denaeyer G, Sanders DR. Virtual scleral lens fitting over large filtering bleb using corneal-scleral topography. Int J Open Access Ophthalmol; 2018;3(1):1-5. 33. Macedo-de-Araújo RJ, van der Worp E, Manuel González-Méijome J, et al. In vivo assessment of the anterior scleral contour assisted by automatic profilometry and changes in conjunctival shape after miniscleral contact lens fitting. J Optom. 2019;12(2):131-40. 34. Ritzmann M, Caroline PJ, Börret R, et al. An analysis of anterior scleral shape and its role in the design and fitting of scleral contact lenses. Cont Lens Anterior Eye. 2018;41(2):205-13. 35. Schornack MM, Vincent SJ, Walker MK. Anatomical and physiological considerations in scleral lens wear: intraocular pressure. Cont Lens Anterior Eye. November 22, 2021. [Epub ahead of print]. 36. Walker MK, Schornack MM, Vincent SJ. Anatomical and physiological considerations in scleral lens wear: eyelids and tear film. Cont Lens Anterior Eye. 2021;44(5):101407. 37. Walker MK, Schornack MM, Vincent SJ. Anatomical and physiological considerations in scleral lens wear: conjunctiva and sclera. Cont Lens Anterior Eye. 2020;43(6):517-28. |


