 |
A 48-year-old Caucasian female presented to the cornea service for an ocular surface check in the setting of ocular graft-vs.-host disease (GVHD), following allogeneic hematopoietic stem cell transplants (HSCT) for recurrent T-cell lymphoma. At the present visit, the patient endorsed new GVHD involvement of the gastrointestinal tract but denied any involvement of other organ systems and was currently off all systemic immunosuppressants. The patient continued to report persistent symptoms of severe ocular dryness, pain, photophobia, visual blur and sensitivity to wind and ambient environments.
Prior treatments, including punctal plugs, cautery of all four puncta, topical Restasis (cyclosporine 0.05%, Allergan) BID, autologous serum tears six to eight times a day, preservative-free artificial tears six times a day, Celluvisc (carboxymethylcellulose sodium 1%, Allergan) gel drops PRN, ocular ointment QHS OU and heat application with hot eye mask provided minimal relief. The patient previously initiated a scleral contact lens fitting with an outside provider but was unable to proceed due to cost. Additional ocular history included excision of a left upper eyelid hemangioma, posterior subscapular cataract in the left eye and nuclear sclerotic cataracts in both eyes post phacoemulsification, with intraocular lens placement post YAG capsulotomy in both eyes as well.
At the present visit, Schirmer scores were 2mm in each eye, and her dense interpalpebral keratopathy, according to the National Eye Institute grading scale, was 7/15 in the right eye and 15/15 in the left. The patient was instructed to continue her current topical lubrication regimen as well as heat application, and a same-day appointment was booked in the specialty contact lens service to initiate a scleral contact lens fitting.
At this visit, the patient was fit into BostonSight scleral lenses, and they were observed to yield excellent comfort in both eyes. They also provided adequate vision with over-refraction (BCVA 20/20 OD/OS) and adequate vault but a loose fit 360° in both eyes. Based on evaluation of this diagnostic lens fitting, a pair of lenses was ordered, and the patient was instructed to return for a dispense visit with insertion and removal training; the patient was also advised to purchase +1.50 OTC readers to be used over the contact lenses.
In several subsequent visits following the initial dispense, the fit was modified slightly by reducing lens diameter and steepening edges to reduce edge lift, with commensurate reduction in lens movement and awareness. Vision was adequate in both eyes—20/20-2 in the right eye, 20/25-2 in the left eye—and continued to improve further, reaching 20/20 in both eyes as keratopathy improved.
Over the course of the next several visits, the corneal surface of both eyes continued to clear and the patient continued to report striking improvement in ocular comfort and vision. She endorsed complete resolution of photophobia and the ability to tolerate ambient environments, like windy conditions, which she was unable to do for several years prior.
Ocular GVHD
A fairly common complication of allogeneic HSCT, GVHD is a major contributor to morbidity and mortality after treatment. In GVHD, classified as either acute or chronic, a dysregulated immune response targets host organ systems, including skin, mouth, eyes, lungs, gastrointestinal (GI) and genitourinary tracts, leading to organ fibrosis and dysfunction.1-4
Ocular GVHD, although a rare manifestation of acute GVHD, is the most common long-term complication with an incidence of about 30% to 60% in patients following HSCT, as well as about 60% to 90% in patients with systemic GVHD involvement.1,2,5-7 Risk factors for ocular GVHD include concurrent skin, mouth, lung, GI or liver GVHD involvement, pre-existing diabetes, Epstein-Barr virus-positive donors, female donors for male recipients and the use of peripheral blood stem cell or bone marrow vs. cord blood transplantation and ethnicity, with Asians and other recipients more likely to develop ocular GVHD compared with Caucasians.2,6-11
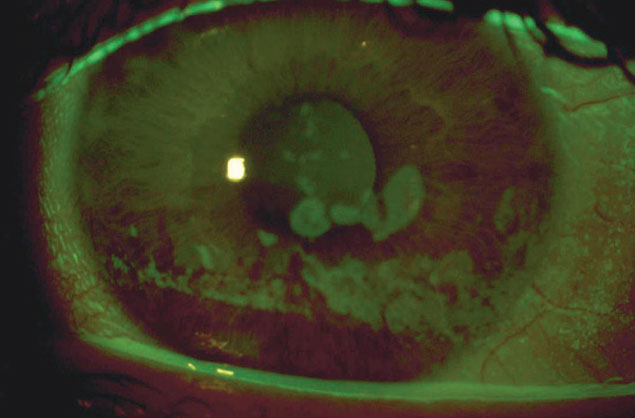 |
|
Coarse epithelial keratopathy: a common indication for scleral lens wear in OGVHD patients. Click image to enlarge. |
In ocular GVHD, dysregulated host immune response damages lacrimal glands, eyelids and the ocular surface via a T cell-mediated inflammatory cascade, resulting in keratoconjunctivitis sicca and cicatricial conjunctivitis.1,12 On clinical presentation, patients may note symptoms consistent with dry eye disease, including ocular dryness, grittiness, pain, irritation, foreign body sensation, redness, photophobia, tearing and/or visual blur; these symptoms often negatively impact quality-of-life and daily activities.1-3, 13-16
Patients with acute-onset ocular GVHD may present with less severe clinical signs, such as conjunctival injection or chemosis. However, many cases present more severe signs, including pseudomembranous or hemorrhagic conjunctivitis, corneal epithelial sloughing, keratitis, filaments or lagophthalmos.1,2,13,17-19
In chronic cases, corneal clinical signs range from punctate keratopathy to epithelial erosions or defects which may rapidly progress to stromal thinning, melting or perforation.1,2,20 Chronic inflammation and fibrosis may lead to development of filamentary keratitis, corneal neovascularization, limbal stem cell deficiency and neurotrophic ulceration.1,2,21,22 Conjunctival and eyelid clinical manifestations include hyperemia or chronic and/or cicatricial conjunctivitis with associated entropion, ectropion, trichiasis, poliosis, keratinization, subepithelial fibrosis, symblepharon, ankyloblepharon eyelid laxity and/or lagophthalmos. Less commonly, pseudomembranes are seen in chronic cases.1,2,18,23
Additional clinical findings may include subtarsal fibrosis of the upper tarsus or superior limbic keratoconjunctivitis, like inflammation due to frictional microtrauma and nasolacrimal duct obstruction.1,24-26 Infiltration and fibrosis of the lacrimal gland and destruction of the meibomian glands contribute to aqueous tear deficiency and unstable tear film, further contributing to ocular surface disease.1,2,27,28 Ocular inflammation in chronic cases may also rarely manifest as episcleritis, anterior or posterior scleritis, anterior or intermediate uveitis, cataracts or serous choroidal detachment.29
Multiple diagnostic criteria exist for ocular GVHD. To diagnose, it is helpful to evaluate tear meniscus height, tear breakup time and tear production by Schirmer testing. Looking at meibomian gland function by means of clinical appearance, manual expression and/or newer modalities such as meibography, tear interferometry, in vivo confocal microscopy or tear film osmolarity are also helpful.1,2 The NIH recommends a baseline ophthalmic examination about 100 days post-transplant and routine monitoring at least every three to 12 months of patients with confirmed ocular GVHD.1,30
Therapeutic Approaches
Systemic immunosuppressants, like corticosteroids, are often employed for GVHD; however, they may fail or become associated with relapse.1,31 Treatment of ocular surface involvement in GVHD aims to improve tear film quality and corneal epithelial integrity, as well as to reduce ocular surface inflammation.1,31 Frequent instillation, at least four times daily, of topical, preservative-free artificial tears is necessary for surface lubrication. More viscous artificial gels and ointments may also be used, particularly at bedtime.1,14,31,32 In cases of low tear production, such as with reduced tear lake height or Schirmer scores, tear production may be increased with topical cyclosporine or lifitegrast. This also increases conjunctival goblet cell density and decreases ocular surface inflammation. Other options include punctal occlusion, either by silicone or collagen plugs or with thermal cauterization.1,29,31,33,34
Blepharitis is managed with eyelid hygiene (e.g., baby shampoo, lid cleansers) and warm compress application twice daily for at least 10 minutes.1,31 Eyelid inflammation may be treated with topical corticosteroids or macrolides (e.g., azithromycin) and systemic tetracyclines (e.g., doxycycline), with application of topical tacrolimus also shown as effective.1,31,35,36 In cases of severe ocular surface disease secondary to GVHD, autologous serum eye drops and/or bandage contact lenses (e.g., sclerals) help to reduce signs and symptoms of dryness.1,31,37,38
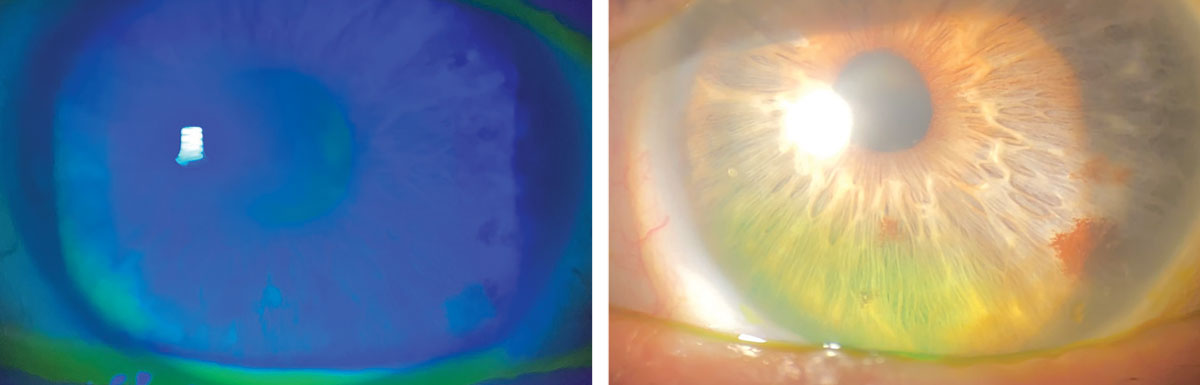 |
|
Significant improvement in coarse epithelial keratopathy following scleral lens wear for OGVHD. Click image to enlarge. |
Partial lateral tarsorrhaphy, amniotic membrane transplantation and limbal epithelial stem cell transplantation have also been reported in severe cases.1,31,39,40 Adjunctive therapy may be used as appropriate, like the use of topical N‐acetylcysteine with present filamentary keratitis, instillation of topical cenegermin or topical insulin for neurotrophic keratopathy, epilation of trichiatic lashes and management of ocular hypertension and cataracts that may result from frequent corticosteroid use.1,31,41
GVHD is increasing in incidence due to advances in HSCT and a rise in HSCT usage for varied hematological conditions.1,2 Given the high prevalence of ocular surface involvement in patients who have undergone this procedure, it is imperative for eyecare providers to recognize the signs and symptoms early on and treat aggressively in order to maintain ocular surface structure and function, as well as preserve patient comfort.
1. Carreno-Galeano JT, Dohlman TH, Kim S, Yin J, Dana R. A review of ocular graft-versus-host disease: pathophysiology, clinical presentation and management. Ocul Immunol Inflamm. 2021;29(6):1190-9. 2. Nair S, Vanathi M, Mukhija R, et al. Update on ocular graft-versus-host disease. Indian J Ophthalmol. 2021;69(5):1038-50. 3. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. 4. Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30-7. 5. Shikari H, Amparo F, Saboo U, Dana R. Onset of ocular graft-versus-host disease symptoms after allogeneic hematopoietic stem cell transplantation. Cornea. 2015;34(3):243-7. 6. Jacobs R, Tran U, Chen H, et al. Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant. 2012;47(11):1470-3. 7. Westeneng AC, Hettinga Y, Lokhorst H, et al. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea. 2010;29(7):758-63. 8. Khan R, Nair S, Seth T, et al. Ocular graft versus host disease in allogenic haematopoetic stem cell transplantation in a tertiary care centre in India. Indian J Med Res. 2015;142(5):543-8. 9. Wang JCC, Teichman JC, Mustafa M, et al. Risk factors for the development of ocular graft-versus-host disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol. 2015;99(11):1514-8. 10. Na KS, Yoo YS, Mok JW, Lee JW, Joo CK. Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015;50(11):1459-64. 11. Uchino M, Ogawa Y, Uchino Y, et al. Comparison of stem cell sources in the severity of dry eye after allogeneic haematopoietic stem cell transplantation. Br J Ophthalmol. 2012;96(1):34-7. 12. Pavletic SZ, Vogelsang GB, Lee SJ. 2014 National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: preface to the series. Biol Blood Marrow Transplant. 2015;21(3):387-88. 13. Saito T, Shinagawa K, Takenaka K, et al. Ocular manifestation of acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2002;75(3):332-4. 14. Espana EM, Shah S, Santhiago MR, Singh AD. Graft versus host disease: clinical evaluation, diagnosis and management. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1257-66. 15. Saboo US, Amparo F, Abud TB, Schaumberg DA, Dana R. Vision-related quality of life in patients with ocular graft-versus-host disease. Ophthalmology. 2015;122(8):1669-74. 16. Sung AD, Chao NJ. Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment. Stem Cells Transl Med. 2013;2(1):25-32. 17. Janin A, Facon T, Castier P, et al. Pseudomembranous conjunctivitis following bone marrow transplantation: immunopathological and ultrastructural study of one case. Hum Pathol. 1996;27(3):307-9. 18. Jabs DA, Wingard J, Green WR, et al. The eye in bone marrow transplantation. III. Conjunctival graft-vs-host disease. Arch Ophthalmol. 1989;107(9):1343-8. 19. Uchino M, Ogawa Y, Kawai M, et al. Ocular complications in a child with acute graft-versus-host disease following cord blood stem cell transplantation: therapeutic challenges. Acta Ophthalmol Scand. 2006;84(4):545-8. 20. Mohammadpour M, Maleki S, Hashemi H, Beheshtnejad AH. Recurrent corneal perforation due to chronic graft versus host disease; a clinicopathologic report. J Ophthalmic Vis Res. 2016;11(1):108-11. 21. Wang Y, Ogawa Y, Dogru M, et al. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant. 2010;45(6):1077-83. 22. Mohammadpour M. Progressive corneal vascularization caused by graft-versus-host disease. Cornea. 2007;26(2):225-6. 23. Balaram M, Rashid S, Dana R. Chronic ocular surface disease after allogeneic bone marrow transplantation. Ocul Surf. 2005;3(4):203-11. 24. Kheirkhah A, Coco G, Satitpitakul V, Dana R. Subtarsal fibrosis is associated with ocular surface epitheliopathy in graft-versus-host disease. Am J Ophthalmol. 2018;189:102-10. 25. Sivaraman KR, Jivrajka RV, Soin K, et al. Superior limbic keratoconjunctivitis-like inflammation in patients with chronic graft-versus-host disease. Ocul Surf. 2016;14(3):393-400. 26. Satchi K, McNab AA. Conjunctival cicatrizing disease presenting with lacrimal obstruction. Orbit. 2016;35(6):321-3. 27. Hwang HS, Ha M, Kim HS, Na KS. Longitudinal analysis of meibomian gland dropout in patients with ocular graft-versus-host disease. Ocul Surf. 2019;17(3):464-9. 28. Tung CI. Graft versus host disease: what should the oculoplastic surgeon know? Curr Opin Ophthalmol. 2017;28(5):499-504. 29. Dietrich-Ntoukas T, Cursiefen C, Westekemper H, et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on clinical practice in chronic GVHD. Cornea. 2012;31(3):299-310. 30. Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984-99. 31. Bruscolini A, Gharbiya M, Sacchetti M, et al. Involvement of ocular surface in graft-versus-host disease: an update from immunopathogenesis to treatment. J Cell Physiol. 2021;236(9):6190-9. 32. Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009;29(6):573-83. 33. Sabti S, Halter JP, Braun Fränkl BC, et al. Punctal occlusion is safe and efficient for the treatment of keratoconjunctivitis sicca in patients with ocular GvHD. Bone Marrow Transplant. 2012;47(7):981-4. 34. Wang Y, Carreno-Galeano JT, Singh RB, Dana R, Yin J. Long-term outcomes of punctal cauterization in the management of ocular surface diseases. Cornea. 2021;40(2):168-71. 35. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders—new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1(Suppl 1):3-47. 36. Abud TB, Amparo F, Saboo US, et al. A clinical trial comparing the safety and efficacy of topical tacrolimus versus methylprednisolone in ocular graft-versus-host disease. Ophthalmology. 2016;123(7): 1449-57. 37. Kojima T, Higuchi A, Goto E, et al. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008;27 Suppl 1:S25-S30. 38. Magro L, Gauthier J, Richet M, et al. Scleral lenses for severe chronic GvHD-related keratoconjunctivitis sicca: a retrospective study by the SFGM-TC. Bone Marrow Transplant. 2017;52(6): 878-82. 39. Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Surv Ophthalmol. 2013;58(3):233-51. 40. Busin M, Giannaccare G, Sapigni L, et al. Conjunctival and limbal transplantation from the same living-related bone marrow donor to patients with severe ocular graft-vs-host disease. JAMA Ophthalmol. 2017;135(10):1123-5. 41. Wang AL, Weinlander E, Metcalf BM, et al. Use of topical insulin to treat refractory neurotrophic corneal ulcers. Cornea. 2017;36(11):1426-8. |


