The modern management of keratoconus can best be characterized by four directives: (1) diagnose early, (2) monitor often, (3) stop progression and (4) improve vision. Significant advances are changing the way we approach each of these four aspects of care. Here, we review many innovations impacting the clinical approach to keratoconus, including advanced diagnostics with corneal topography, optical coherence tomography (OCT) and wavefront aberrometry. New approaches to therapy include corneal collagen crosslinking (CXL) and specialty contact lenses, to name a few.
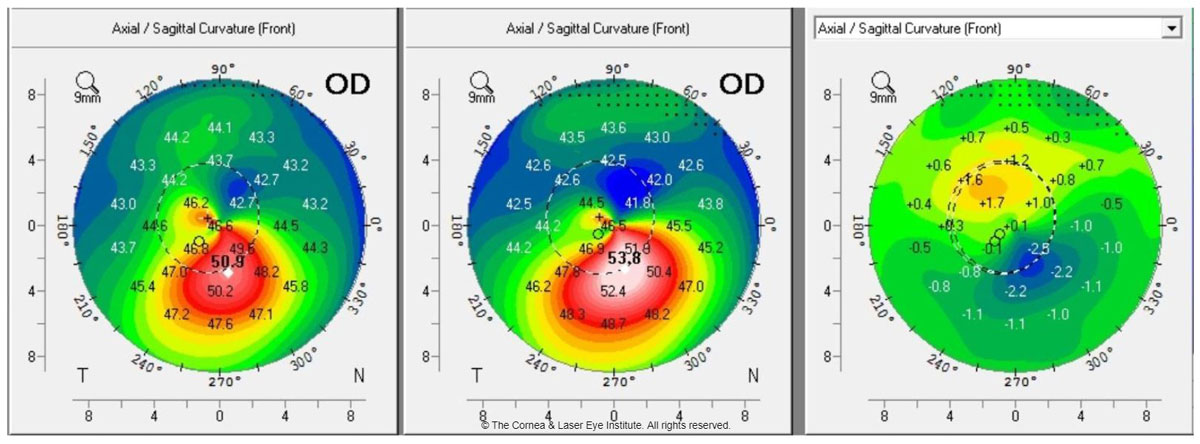 |
| Fig. 1a. These axial curvature topography maps display differences (right) in pre-op curvature (center) to one-year post-op curvature (left). This cornea shows flattening of the corneal apex, consistent with the dioptric change reported in the literature one year after standard CXL. Photo: John Gelles, OD. Click image to enlarge. |
Diagnose and Monitor
Our ability to diagnose and monitor keratoconus has improved tremendously in recent decades. The traditional Amsler-Krumeich keratoconus classification system, established in 1946, is based on a combination of pachymetry, slit lamp findings, central keratometry and refraction.1 However, today’s diagnostic workup includes far more than those four metrics of the 1940s. No currently agreed upon classification system has been established to account for the rapid evolution in device technology, but the traditional Amsler-Krumeich classification system is long due for an update.
Here at the Cornea and Laser Eye Institute (CLEI), we analyzed approximately 1,200 eyes with keratoconus to understand baseline characteristics and explore findings that may suggest the need for further testing. The preliminary analysis suggests the Amsler-Krumeich exaggerates the level of myopia and, because it uses central keratometry, misses the true severity of the disease. The data indicates these patients are in need of further testing to rule out keratoconus.
The advent of corneal topography provided new metrics for reviewing cornea curvature and symmetry.2 We now rely on several topographic metrics to diagnose keratoconus. Some of the earliest suggested are:3,4
- Keratometry values greater than 47.0D.
- Axis skew between the steepest superior and inferior semi-meridians of greater than 20° with greater than 1.5D of corneal astigmatism.
- Inferior to superior (I-S)keratometry value differences greater than 1.4D on an axial curvature map.
These individual metrics then led to the development of multifactor metrics that can provide higher levels of diagnostic specificity and sensitivity.5-8
Corneal tomography added information about global corneal pachymetry and anterior and posterior corneal elevation. Diagnostic metrics derived from tomography include: thinnest pachymetry less than 500µm, anterior elevation greater than 10µm to 15µm and posterior elevation greater than 15µm to 20µm.9-11
In addition, an impressive array of device-specific multifactor algorithms are available for early detection, monitoring and classification, all of which are highly sensitive and specific for diagnosing keratoconus and monitoring for progression.7,12-16
Clinicians can now analyze individual corneal layers with ultra high-resolution ultrasound and OCT. Epithelial remodeling in keratoconus, thinning of the epithelium over the cone and epithelial thickening around the base have all been well documented and have diagnostic value.17-19
One study suggests maximum and minimum epithelial thickness greater than 16.3µm and pattern standard deviations of greater than 0.057 are highly specific and sensitive for early keratoconus detection.20 When comparing normal corneas with forme fruste keratoconus corneas, researchers found significant epithelial thickness differences despite having no differences in corneal topography.21 This thinning also affects Bowman’s layer. Other researchers used an enhanced OCT algorithm to visualize Bowman’s layer and found statistically significant inferior thinning compared with a normal cornea.22 These same changes in Bowman’s layer thickness are found in global pachymetry measurements.23
The missing link has always been in measuring corneal biomechanics, another area of diagnostic innovation. Infrared sensor-based, waveform-derived biomechanical data can differentiate normal from grossly abnormal. Outside of the United States, highly sensitive and specific data has been derived from Scheimpflug-based biomechanical data and has become common practice for evaluating corneal biomechanics.24,25 This technology captures ultra high-speed video of the corneal deformation under applanation and analyzes the amplitudes and temporal qualities.
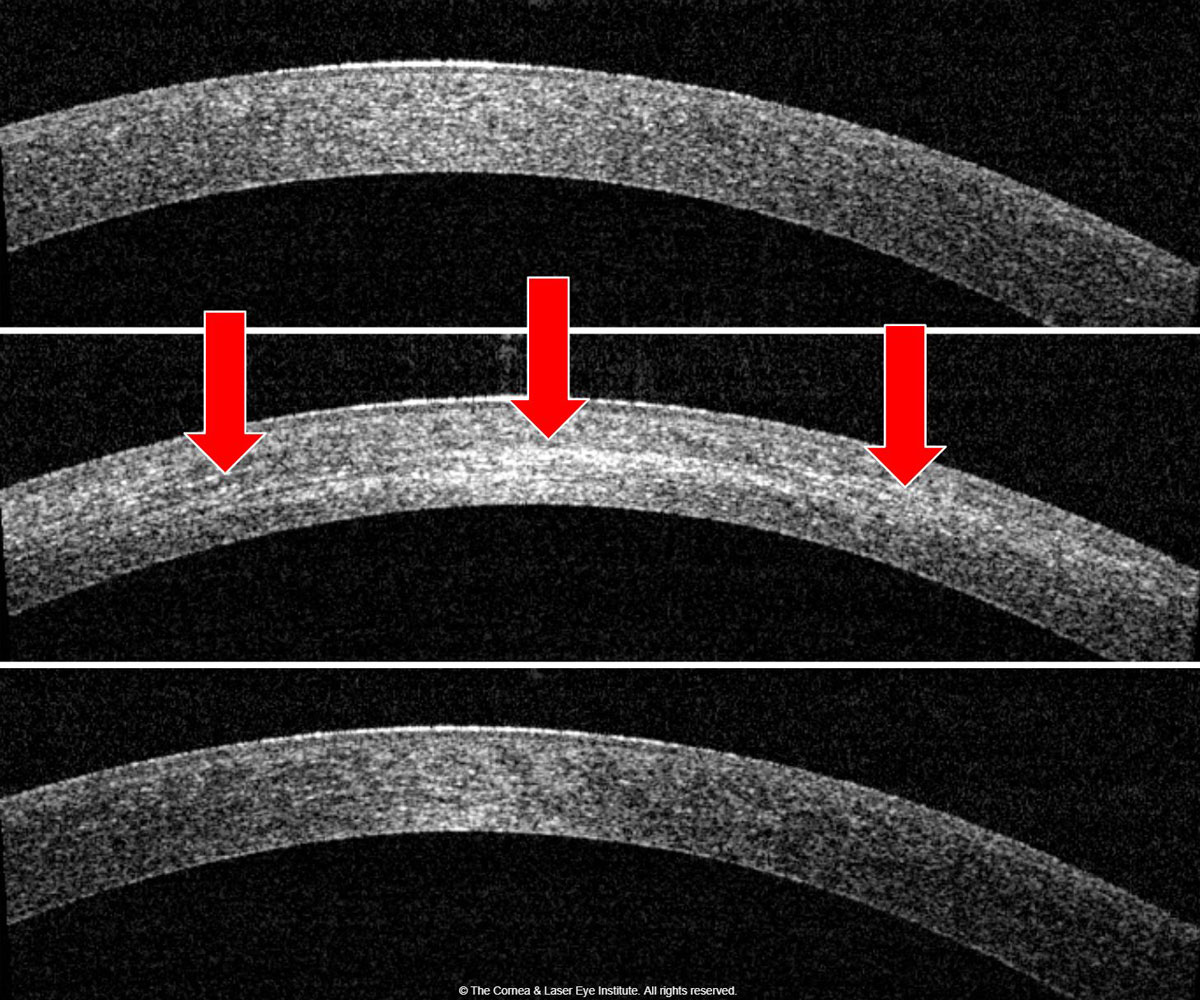 |
| Fig. 1b. Corneal OCT shows the development of corneal haze from the pre-op (top) to one month post-op (middle). Note the presence of the demarcation line (red arrows), then resolution of corneal haze from the one-month post-op to the 12-month post-op (bottom). Photo: John Gelles, OD. Click image to enlarge. |
Two new tools, OCT elastography and brillouin, have mostly been used in laboratory and research settings, although both are currently under development for clinical applications. Research with OCT elastography shows the depth-related strength of cornea tissue; in normal corneas, anterior strength is higher than the posterior. By contrast, keratoconus corneas have a loss of anterior biomechanical strength.26
Waveform-derived biomechanical metrics in their current forms give global corneal biomechanics and are not completely location specific; therefore, a weakness in a specific point of the cornea cannot be pinpointed; however, it can with elastography and brillouin microscopy. This has become particularly important now that research shows CXL can increase anterior corneal strength.27
Brillouin derives focal metrics throughout the cornea by analyzing photonic shifts and can be targeted to any focal point in the corneal tissue. Researchers have found that the weakness of the corneal tissue in keratoconus is focal to the cone location, while the rest of the cornea displays properties like those of a normal cornea.28-30 However, with measurement of such small shifts, patient movement can affect brillouin results.
Wavefront aberrometry is another helpful tool when evaluating for keratoconus. The type and extent of higher-order aberrations can be used as a proxy to detect early optical changes in keratoconus. Studies show a significant difference between normal, suspect and keratoconic corneas in total, 3rd, 4th and 5th orders of aberrations, and total, coma (vertical coma, specifically) and trefoil have the most statistically significant differences.31,32
On the Rise or Better Identified?The diagnostic evolution in keratoconus may have led, in part, to the increased prevalence of keratoconus reported over the past several years. The commonly quoted prevalence, approximately 1:2,000, came from a 1998 review and has been challenged by more recent studies.1 Now, research suggests an approximate prevalence of keratoconus of 1:725 worldwide.2 More specific population studies suggest a prevalence of 1:375 in the Netherlands, 1:191 in New Zealand adolescents, 1:45 in a sub-analysis of the New Zealand Maori population and 1:84 in Australia, the last of which was based on Scheimpflug tomography.3-5
|
In combined topographer and aberrometer devices, the ray trace–derived corneal aberrometry can be compared with the total ocular aberration to determine the source, whether it’s the corneal surface or an internal aberration. One study noted that the combination of topography and aberrometry was more than 95% sensitive and specific.33
Others found the specific combination of I-S value from topography and vertical coma from aberrometry was highly statistically significant.34
Although combination metrics can help clinicians better detect and monitor keratoconus, many devices are unable to communicate with or combine data from other devices. New devices available outside the United States are able to combine the data derived from Scheimpflug tomography with Scheimpflug biomechanics to review risk factors and provide an ectasia risk score.35
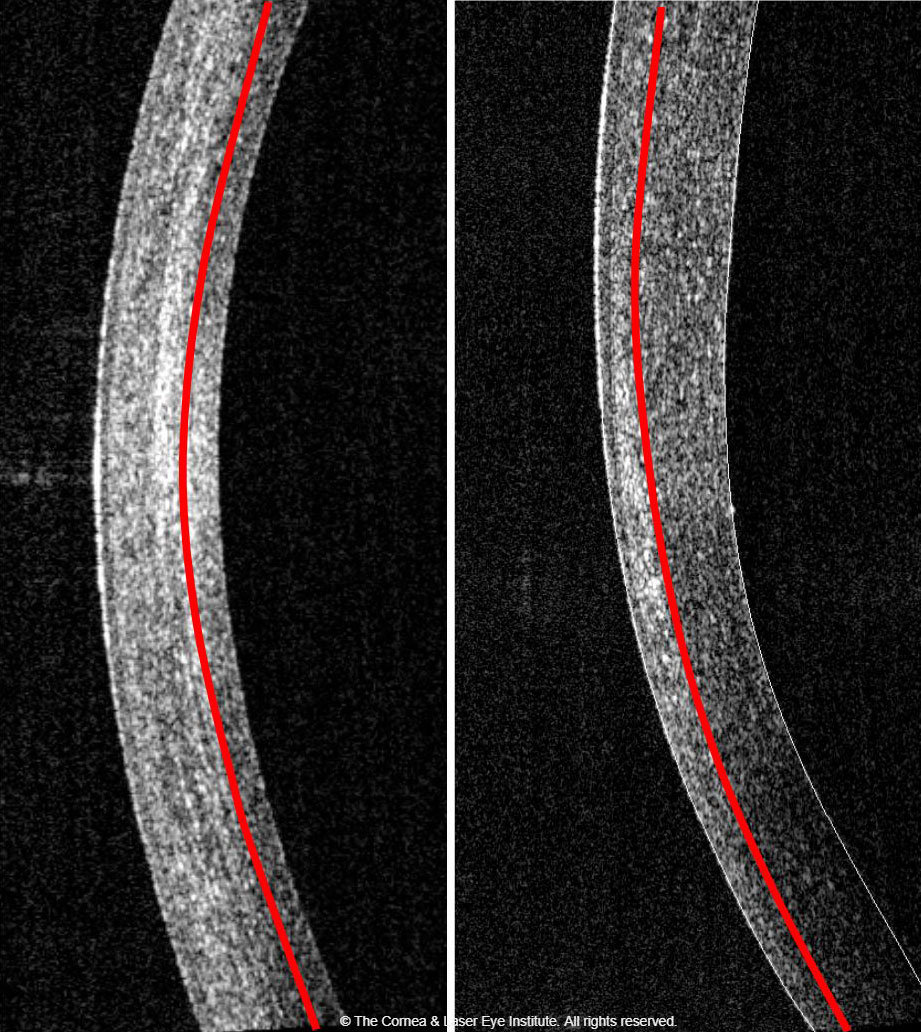 |
| Fig. 2. Comparison of corneal haze and demarcation line depth at the one-month follow-up. The standard CXL procedure (left) produces a more profound haze with a deeper demarcation line depth than the epi-on CXL procedure (right). Photo: John Gelles, OD. Click image to enlarge. |
Using the power of artificial intelligence, researchers developed an algorithm capable of diagnosing and classifying keratoconus with high sensitivity and specificity, without being device- or manufacturer-specific—meaning it could be applied to any device.36 Researchers have also used a convolutional neural network to analyze tomography and found 100% sensitivity and specificity for classification of keratoconus.37
Genetic testing for keratoconus in a clinically impactful capacity, while in its infancy, is an exciting possibility. Researchers found an association with chromosome 5 and the lysyl oxidase (LOX) gene that is responsible for the production of lysyl oxidase, which initiates the natural crosslinking of the corneal collagens.38-40 Studies show decreased levels of LOX in keratoconus corneas compared with the normal cornea.41
Recently, investigators discovered a genome-wide significant loci that met statistical significance required by genetic standards, located on chromosome 11 in the region of PNPLA2.42 More data will emerge as the commercially available genetic test becomes more widely used, and researchers may find more associations. In the future, this could lead to targeted genetic treatment.
Stop Progression
CXL, mediated by riboflavin and ultraviolet (UV) light illumination, has been standard of care in Europe to slow the progression of keratoconus since researchers documented its efficacy in 2003.43
In the United States, experience with the procedure began in 2008 with Phase III clinical trials using the standard protocol (central 9mm of epithelium removed followed by a 30-minute riboflavin soak and 30-minute illumination of 365nm UV wavelength at 3mW/cm2 for a total energy of 5.4J/cm2). The treatment was effective in slowing the progression of keratoconus, resulting in the 2016 FDA-approval of the KXL UV illumination device (Glaukos) and two riboflavin formulations (Photrexa and Photrexa viscous, Glaukos).28,44
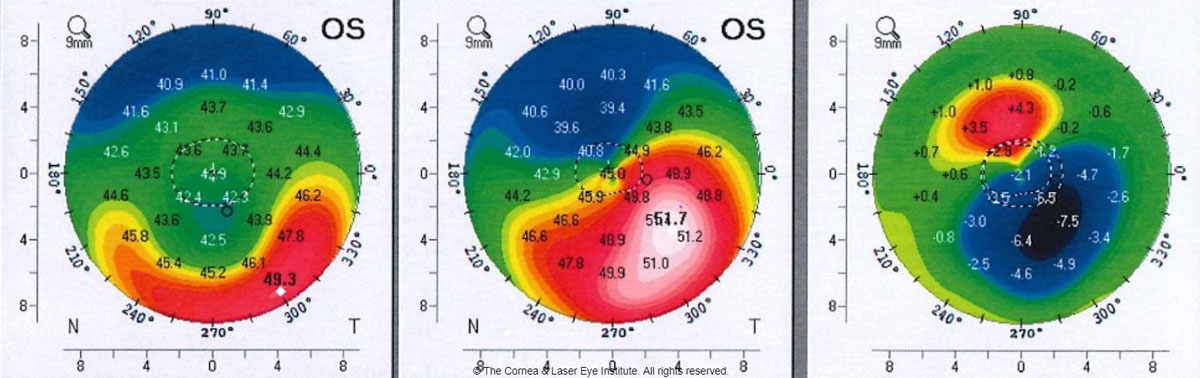 |
| Fig 3. These axial curvature topography maps show the differences (right) in pre-op curvature (center) to six months post-op TGPRK (left) curvature. Photo: John Gelles, OD. Click image to enlarge. |
Many manuscripts have been published by CLEI describing the clinical time course and efficacy of the standard CXL treatment.29,30,45-51 The important observations in the treatment group focused on visual acuity, maximum keratometry and transient corneal haze with a demarcation line.29,45,46 We found that these metrics are initially worse at the one-month post-op and improve, on average, to better than baseline over the first year (Figures 1a and 1b). These studies also show an excellent safety profile (only one severe adverse event), an improvement in subjective patient-reported vision and no significant change (i.e., damage or loss of cell density) to the endothelial cells.44,47
Many variations to the procedure are continuously under development, and researchers are exploring innovations within each of three necessary components of crosslinking: riboflavin, UV light and oxygen. For instance, we recently published a study looking at different osmolarities to keep corneal thickness more consistent throughout the procedure. Our hypotonic riboflavin study showed no difference in efficacy when comparing the standard protocol with dextran-containing riboflavin but did show the ability to maintain a thicker stroma during UV illumination.52
One technique of interest is transepithelial, or epi-on, CXL. The current US literature shows a less robust effect compared with the standard CXL procedure.53,54 Our center’s data shows less corneal flattening after the procedure and less haze formation with a shallower demarcation line (Figure 2).54-56 Still, the procedure doesn’t seem to create the temporary worsening of outcome metrics at the one-month mark. This may be due to an intact epithelium, which does not undergo epithelial remodeling, or simply because it is a less effective treatment.
Various riboflavin formulations have been studied internationally, and most include additives to the riboflavin, such as benzalkonium chloride, to create faster and more complete penetration through intact epithelium.52,54 Others have changed riboflavin concentrations and additives to change the osmolarity.
The riboflavin delivery method is under investigation as well, and researchers are working on ways to soak the cornea by a fluid retention ring well or pledget, methods to speed up the saturation process (e.g., with iontophoresis) and ways to keep the epithelium anchored but disrupted, such as epithelium disruptor instruments or femto laser microchannels, for easier penetration.57,58
One recently developed delivery system increases riboflavin retention time on the corneal surface, shortening time to full corneal saturation, and another uses a scleral lens filled with riboflavin that has an internal illumination system to complete the CXL procedure.59,60 CLEI is currently analyzing a method of scleral lens-based riboflavin delivery compared with traditionally applied drops.
As for UV illumination, most innovations focus on accelerating the procedure by increasing the power and reducing the illumination duration. While these accelerated treatments maintain the overall energy dose of 5.4J/cm2, they produce shallower stromal demarcation lines and seem to be less effective in some studies.61,62
Pulsing the UV illumination remains under investigation and allows for more oxygen to regenerate, which is a vital and rate-limiting component in the production of crosslinks.63
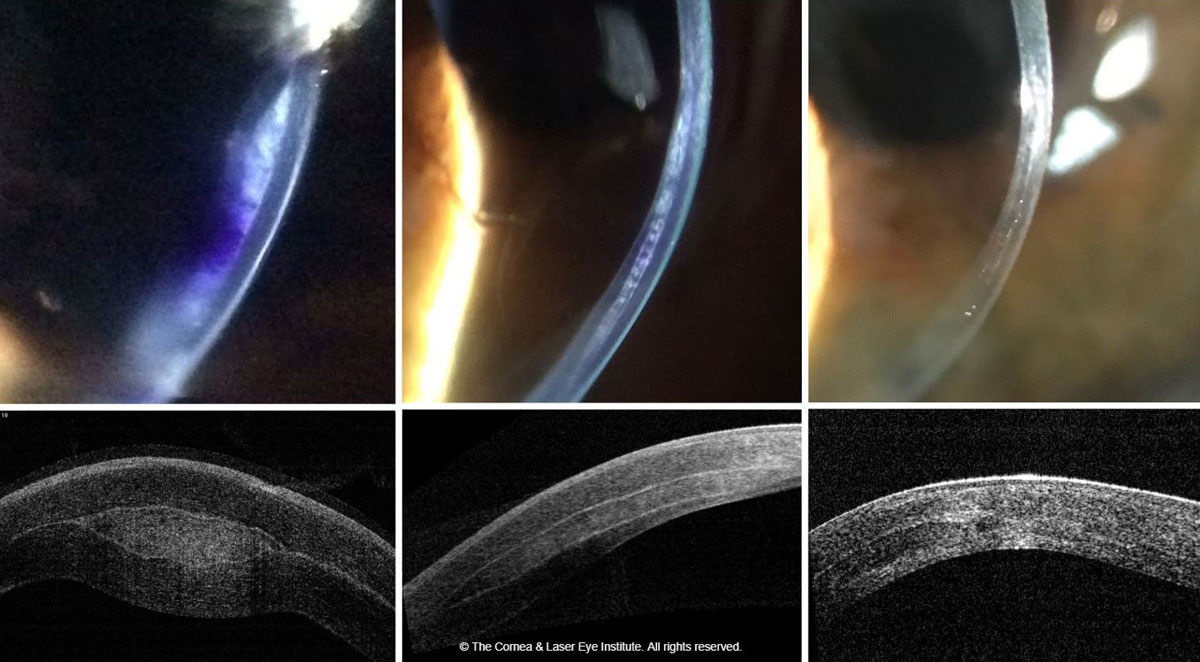 |
| Fig. 4. These images show the optic section and corneal OCT of post-op allograft corneal tissue implantation for keratoconus, immediately post-op (left), one month post-op (middle) and 18 months post-op (right). Photo: John Gelles, OD. Click image to enlarge. |
Another modification is the delivery of a topography guided CXL treatment with multi-energy focal UV illumination. This approach more focally treats the weaker cone region of the cornea by customizing the position, zone and energy applied, thus creating focally deep demarcation lines and greater corneal curvature flattening.64
In the United States, an ongoing Phase III epi-on CXL clinical trial combines several of these new approaches. The procedure uses riboflavin formulated to penetrate intact the epithelium, a pulsed and accelerated application of UV light and oxygen supplementation provided by specialized goggles. Though formal data is not yet available, researchers reported a prominent corneal curvature change, more oxygen availability to the cornea and increased tissue strength ex-vivo.65
Treatment with CXL can be difficult with thin corneas; thus, researchers are exploring techniques to make the procedure possible for these patients. The use of a non UV-blocking contact lens soaked in riboflavin and corneal tissue lenticule on-lays to create artificial thickness are two possibilities.66,67 Customized time and fluence is another variation that uses the demarcation lines to create a time-related model whereby less time will result in a shallower treatment for thinner corneas.68,69
Still others have used rose bengal and green light illumination to induce crosslinking, known as RGX. This treatment results in a very shallow demarcation line and a small increase in anterior corneal strength based on brillouin.55,70
Even newer CXL treatments, such as dual photon femtosecond laser, forgo photosensitizers altogether. Femtosecond lasers that use a sub-cavitation level of energy crosslink tissue rather than cleave it.71 The benefits could include improved precision and reduced treatment duration.
Another non-photosensitizing treatment option is the use of topical LOX supplementation. In a Phase I/II-a study, investigators found approximately 1.00D of flattening in the treatment group, while the control group progressed by approximately 0.46D.72
Improve Vision
The opportunity to strengthen the cornea with CXL has opened new avenues of surgical keratoconus management. In addition to traditional corneal transplant, such as penetrating keratoplasty or deep anterior lamellar keratoplasty, subtractive and additive procedures are showing promise, as are custom scleral contact lenses.
Subtractive procedures. Excimer and femtosecond laser technology modifies corneal shape by subtracting or removing corneal tissue. During the evolution of these lasers and procedures, anatomical and optical data derived from topography and aberrometry helped to create custom ablation profiles to optimize refractive results in normal corneas.
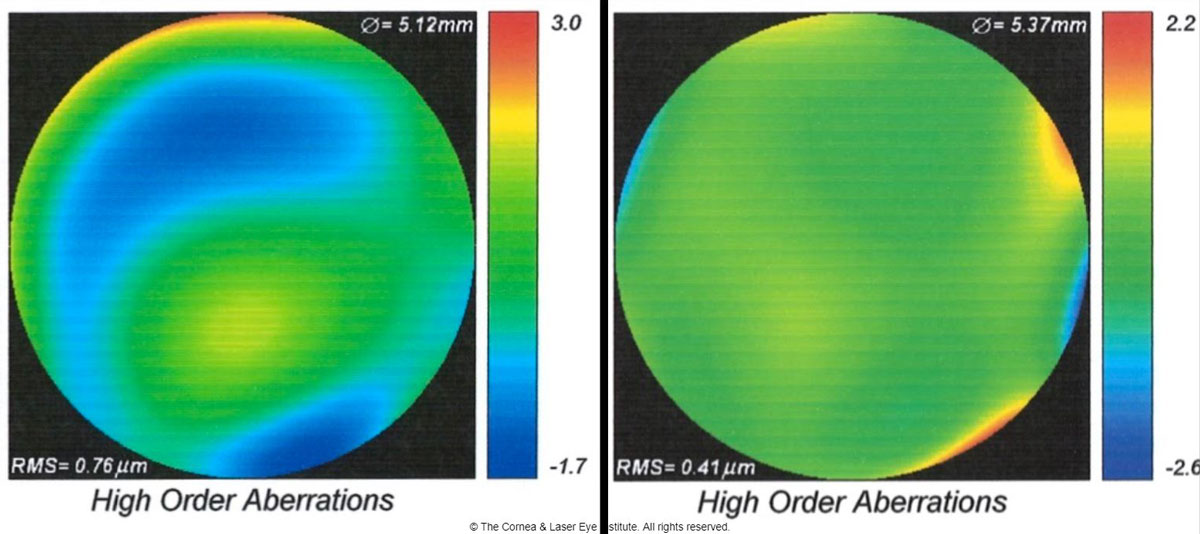 |
| Fig 5. These are the total higher-order aberration maps of an eye of a patient with severe keratoconus while wearing a scleral lens. The map of total aberrations while wearing the final scleral lens corrected with spherical optics and a plano over-refraction (left) is compared with the final customized higher-order aberration correcting optics (right). A 46% reduction of total higher-order root mean square values was observed, translating to one to two lines of visual acuity improvement. Photo: John Gelles, OD. Click image to enlarge. |
Many of these same technologies can be applied to the keratoconic cornea to re-sculpt corneal curvature, with the goal of improving symmetry and total aberrations and creating partial refractive correction.
Guided ablations, whether topographically or wavefront-guided, have been pioneered outside of the United States where more customizable ablations have been available for some time. Research shows improvements in curvature and visual acuity with wavefront-guided ablations combined with CXL, and even ten-year data shows stability of keratometry and visual acuity after concurrent CXL with topography guided ablations.73,74
In the United States, patients treated with CXL first and then topography guided photorefractive keratectomy (PRK) several months later experienced improved topography measures and visual acuity.75
In our center, we found transepithelial topography-guided PRK (TGPRK) is capable of improving uncorrected visual acuity by approximately four lines and one to two lines of best-corrected spectacle visual acuity (Figure 3).
Additionally, this can be used in patients after intracorneal ring segments (ICRS) to improve outcomes and enhance visual acuity postoperatively. Furthermore, a sequential treatment with TGPRK first, for the purpose of improving corneal symmetry, can allow for improved outcomes with intraocular and implantable collamer lens-based procedures by allowing for improved biometry measurement and decreased corneal aberrations postoperatively.
Additive procedures. Recently, randomized prospective trials out of our center and abroad show ICRS procedures can be safely performed concurrently with CXL and that single segments seem best.76 However, the corneal biocompatibility of PMMA placed at 70% to 80% depth in the cornea can occasionally present a problem. We found a 2.5% rate of ICRS explantation due to medical complications.77 Thus, allograft and even xenograft corneal tissue inlays may be the future materials of choice.78-81
Since 2016, CLEI has had an open clinical trial for implantation of e-beam sterilized allogeneic stroma, which is shaped on the femtosecond and excimer lasers and implanted into the cornea via femto laser pocket or channel. This work has shown large changes in corneal curvature (flattening of up to approximately 20.0D) and excellent biocompatibility (Figure 4).
Contact lenses. These innovations, especially scleral lenses, have progressed by leaps and bounds. Profilometry devices, capable of measuring the ocular surface up to 22mm in coverage, are providing a deeper understanding of the contour of the scleral surface, with one study suggesting the scleral contour is mostly asymmetric.82
These same devices are now enabling freeform lenses. At CLEI, we analyzed approximately 560 eyes fit with scleral lenses and found that it took fewer lenses to achieve a final fit with impression-based lenses compared with profilometry/scan-based lenses and diagnostically fit lenses. Though this is prepublication data, it shows the trend that technology can improve the fitting process.
Research shows sclerals with optimized optical profiles can improve high and low contrast visual acuity with the addition of front surface eccentricity.83 Higher-order aberration correction on scleral lenses has been studied as well. The literature shows a 43% reduction in higher-order root mean square values with wavefront-guided optics for patients with ectasia.84 Others found these values decreased from 1.17µm to 0.37µm, a 68% reduction, and a mean of 1.9 lines of visual improvement.85
This approach to scleral lens design is starting to make its way into clinical practice and is showing promise at our center (Figure 5).
Advances in diagnostic imaging and therapy have already changed the keratoconus care paradigm for the better with earlier diagnosis and more effective treatment. As newer innovations move from the lab to the clinic, they will further improve our understanding of the disease process and help us better care for these patients. Until then, today’s technologies give practitioners the tools they need to diagnose early, monitor often, stop progression, and improve vision.
Dr. Gelles is the director of the specialty contact lens division at the Cornea and Laser Eye Institute (CLEI)-Hersh Vision Group and the CLEI Center for Keratoconus in Teaneck, NJ. He is also a clinical assistant professor in the Department of Ophthalmology at Rutgers New Jersey Medical School in Newark, NJ.
Disclosures: Glaukos, BostonSight, Bausch + Lomb, Gas Permeable Lens Institute, International Keratoconus Academy, Scleral Lens Education Society, Synergeyes, Visionary Optics.
Dr. Hersh is the director of the CLEI–Hersh Vision Group and CLEI Center for Keratoconus. He is also a clinical professor in the Department of Ophthalmology at Rutgers New Jersey Medical School and a visiting research collaborator in the Department of Aerospace and Mechanical Engineering at Princeton University in Princeton, NJ.
Disclosures: Glaukos.
Dr. Greenstein is a cornea and refractive surgeon at the CLEI-Hersh Vision Group and CLEI Center for Keratoconus. He is also a clinical assistant clinical professor in the Department of Ophthalmology at Rutgers New Jersey Medical School.
Disclosures: none.
1. Amsler M. Classic keratocene and crude keratocene; Unitary arguments. Ophthalmologica. 1946;111:96-101. 2. Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984;25:1426-35. 3. Wilson SE, Lin DT, Klyce SD. Corneal topography of keratoconus. Cornea. 1991;10:2-8. 4. Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995;11:371-9. 5. Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. 1999;25:1327-35. 6. Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated keratoconus screening with corneal topography analysis. Invest Ophthalmol Vis Sci. 1994;35:2749-57. 7. Zhang X, Munir SZ, Sami Karim SA, Munir WM. A review of imaging modalities for detecting early keratoconus. Eye (Lond). July 16, 2020. [Epub ahead of print]. 8. McMahon TT, Szczotka-Flynn L, Barr JT, et al. A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS). Cornea. 2006;25:794-800. 9. Martinez-Abad A, Pinero DP. New perspectives on the detection and progression of keratoconus. J Cataract Refract Surg. 2017;43:1213-27. 10. Uçakhan Ö, Cetinkor V, Özkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg. 2011;37:1116-24. 11. Miháltz K, Kovács I, Takács A, Nagy ZZ. Evaluation of keratometric, pachymetric, and elevation parameters of keratoconic corneas with pentacam. Cornea. 2009;28:976-80. 12. Duncan JK, Belin MW, Borgstrom M. Assessing progression of keratoconus: novel tomographic determinants. Eye Vis (Lond). 2016;3:6. 13. Belin MW, Duncan JK. [Keratoconus: The ABCD grading system]. Klin Monbl Augenheilkd. 2016;233:701-7. 14. Moshirfar M, Motlagh MN, Murri MS, et al. Galilei corneal tomography for screening of refractive surgery candidates: a review of the literature, Part II. Med Hypothesis Discov Innov Ophthalmol. 2019;8:204-18. 15. Motlagh MN, Moshirfar M, Murri MS, et al. Pentacam(R) corneal tomography for screening of refractive surgery candidates: a review of the literature, Part I. Med Hypothesis Discov Innov Ophthalmol. 2019;8:177-203. 16. Shetty R, Rao H, Khamar P, et al. Keratoconus screening indices and their diagnostic ability to distinguish normal from ectatic corneas. Am J Ophthalmol. 2017;181:140-8. 17. Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25:604-10. 18. Reinstein DZ, Gobbe M, Archer TJ, et al. Epithelial, stromal, and total corneal thickness in keratoconus: three-dimensional display with artemis very-high frequency digital ultrasound. J Refract Surg. 2010;26:259-71. 19. Silverman RH, Urs R, RoyChoudhury A, et al. Combined tomography and epithelial thickness mapping for diagnosis of keratoconus. Eur J Ophthalmol. 2017;27:129-34. 20. Li Y, Chamberlain W, Tan O, et al. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J Cataract Refract Surg. 2016;42:284-95. 21. Temstet C, Sandali O, Bouheraoua N, et al. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract Refract Surg. 2015;41:812-20. 22. Abou Shousha M, Perez VL, Fraga Santini Canto AP, et al. The use of Bowman’s layer vertical topographic thickness map in the diagnosis of keratoconus. Ophthalmology. 2014;121:988-93. 23. Pircher N, Beer F, Holzer S, et al. Large field of view corneal epithelium and Bowman’s layer thickness maps in keratoconic and healthy eyes. Am J Ophthalmol. 2020;209:168-77. 24. Steinberg J, Frings A, Mousli A, et al. New Scheimpflug dynamic in vivo curve analyses to characterize biomechanical changes of the cornea after cross-linking for progressive keratoconus. J Refract Surg. 2016;32:34-9. 25. Vinciguerra R, Romano V, Arbabi EM, et al. In vivo early corneal biomechanical changes after corneal cross-linking in patients with progressive keratoconus. J Refract Surg. 2017;33:840-6. 26. Vinciguerra R, Romano V, Arbabi EM, et al. In vivo early corneal biomechanical changes after corneal cross-linking in patients with progressive keratoconus. J Refract Surg. 2017;33:840-6. 27. Blackburn BJ, Gu S, Ford MR, et al. Noninvasive assessment of corneal crosslinking with phase-decorrelation optical coherence tomography. Invest Ophthalmol Vis Sci. 2019;60:41-51. 28. Hersh PS, Stulting RD, Muller D, et al. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology. 2017;124:1475-84. 29. Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36:2105-14. 30. Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:1282-90. 31. Lim L, Wei RH, Chan WK, Tan DT. Evaluation of higher order ocular aberrations in patients with keratoconus. J Refract Surg. 2007;23:825-8. 32. Kosaki R, Maeda N, Bessho K, et al. Magnitude and orientation of Zernike terms in patients with keratoconus. Invest Ophthalmol Vis Sci. 2007;48:3062-8. 33. Saad A, Gatinel D. Combining placido and corneal wavefront data for the detection of forme fruste keratoconus. J Refract Surg. 2016;32:510-6. 34. Jafri B, Li X, Yang H, Rabinowitz YS. Higher order wavefront aberrations and topography in early and suspected keratoconus. J Refract Surg. 2007;23:774-81. 35. Ferreira-Mendes J, Lopes BT, Faria-Correia F, et al. Enhanced ectasia detection using corneal tomography and biomechanics. Am J Ophthalmol. 2019;197:7-16. 36. Jimenez-Garcia M, Ni Dhubhghaill S, Koppen C, et al. Baseline findings in the Retrospective Digital Computer Analysis of Keratoconus Evolution (REDCAKE) Project. Cornea. June 10, 2020. [Epub ahead of print]. 37. Zéboulon P, Debellemanière G, Bouvet M, Gatinel D. Corneal topography raw data classification using a convolutional neural network. Am J Ophthalmol. 2020;219:33-9. 38. Tang YG, Rabinowitz YS, Taylor KD, et al. Genomewide linkage scan in a multigeneration Caucasian pedigree identifies a novel locus for keratoconus on chromosome 5q14.3-q21.1. Genet Med. 2005;7:397-405. 39. Bisceglia L, De Bonis P, Pizzicoli C, et al. Linkage analysis in keratoconus: replication of locus 5q21.2 and identification of other suggestive Loci. Invest Ophthalmol Vis Sci. 2009;50:1081-6. 40. Bykhovskaya Y, Li X, Epifantseva I, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012;53:4152-7. 41. Dudakova L, Liskova P, Trojek T, et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res. 2012;104:74-81. 42. McComish BJ, Sahebjada S, Bykhovskaya Y, et al. Association of genetic variation with keratoconus. JAMA Ophthalmol. 2020;138:174-81. 43. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620-7. 44. Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124:1259-70. 45. Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:691-700. 46. Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:149-60. 47. Brooks NO, Greenstein S, Fry K, Hersh PS. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38:615-9. 48. Greenstein SA, Fry KL, Hersh MJ, Hersh PS. Higher-order aberrations after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38:292-302. 49. Greenstein SA, Fry KL, Hersh PS. In vivo biomechanical changes after corneal collagen cross-linking for keratoconus and corneal ectasia: 1-year analysis of a randomized, controlled, clinical trial. Cornea. 2012;31:21-5. 50. Greenstein SA, Fry KL, Hersh PS. Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg. 2012;28:397-405. 51. Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: implications for patient selection. J Cataract Refract Surg. 2013;39:1133-40. 52. Rosenblat E, Hersh PS. Intraoperative corneal thickness change and clinical outcomes after corneal collagen crosslinking: Standard crosslinking versus hypotonic riboflavin. J Cataract Refract Surg. 2016;42:596-605. 53. Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal crosslinking without epithelial removal. J Cataract Refract Surg. 2018;44:1363-70. 54. Hersh PS, Lai MJ, Gelles JD, Lesniak SP. Transepithelial corneal crosslinking for keratoconus. J Cataract Refract Surg. 2018;44:313-22. 55. Lesniak SP, Hersh PS. Transepithelial corneal collagen crosslinking for keratoconus: six-month results. J Cataract Refract Surg. 2014;40:1971-9. 56. M JL, Greenstein SA, Gelles JD, Hersh PS. Corneal haze after transepithelial collagen cross-linking for keratoconus: a Scheimpflug densitometry analysis. Cornea. 2020;39:1117-21. 57. Cherfan D, Verter EE, Melki S, et al. Collagen cross-linking using rose bengal and green light to increase corneal stiffness. Invest Ophthalmol Vis Sci. 2013;54:3426-33. 58. Bradford S, Mikula E, Xie Y, et al. Enhanced transepithelial riboflavin delivery using femtosecond laser-machined epithelial microchannels. Transl Vis Sci Technol. 2020;9:1. 59. Godin M, Hansen M, Reeves S, Hardten D. Use of the keraflow device in epithelium on corneal collagen crosslinking. ASCRS 2019. 60. Batlle L, Rivera CD. Trans-epithelial (epithelium-on) corneal collagen crosslinking using a novel ultraviolet light-emitting contact lens device. 2020. ASCRS 2020. 61. Wen D, Li Q, Song B, et al. Comparison of standard versus accelerated corneal collagen cross-linking for keratoconus: a meta-analysis. Invest Ophthalmol Vis Sci. 2018;59:3920-31. 62. Spadea L, Di Genova L, Tonti E. Corneal stromal demarcation line after 4 protocols of corneal crosslinking in keratoconus determined with anterior segment optical coherence tomography. J Cataract Refract Surg. 2018;44:596-602. 63. Mazzotta C, Traversi C, Paradiso AL, et al. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: one-year results. J Ophthalmol. 2014;2014:604731. 64. Mazzotta C, Moramarco A, Traversi C, et al. accelerated corneal collagen cross-linking using topography-guided uv-a energy emission: preliminary clinical and morphological outcomes. J Ophthalmol. 2016;2016:2031031. 65. Hill J, Liu C, Deardorff P, et al. Optimization of oxygen dynamics, UV-A delivery, and drug formulation for accelerated epi-on corneal crosslinking. Curr Eye Res. 2020;45:450-8. 66. Jacob S, Kumar DA, Agarwal A. Reply: To PMID 24972403. J Refract Surg. 2014;30:728-9. 67. Cagini C, Riccitelli F, Messina M, et al. Epi-off-lenticule-on corneal collagen cross-linking in thin keratoconic corneas. Int Ophthalmol. August 13, 2020. [Epub ahead of print]. 68. Caruso C, Epstein RL, Ostacolo C, et al. Customized corneal cross-linking-a mathematical model. Cornea. 2017;36:600-4. 69. Hafezi F, Gilardoni F, Kling S, et al. Results of a prospective study using individualized fluence corneal-cross-linking in ultra-thin corneas: the sub400 protocol. ESCRS 2020. 70. Germann JA, Martinez-Enriquez E, Martinez-Garcia MC, et al. Corneal collagen ordering after in vivo rose bengal and riboflavin cross-linking. Invest Ophthalmol Vis Sci. 2020;61:28. 71. Guo Y, Wang C, Celi N, Vukelic S. Femtosecond laser collagen cross-linking without traditional photosensitizers. SPIE 2015. 72. Molokhia S, Muddana SK, Hauritz H, et al. IVMED 80 eye drops for treatment of keratoconus in patients -Phase 1/2a. Invest Ophthalmol Vis Sci. 2020;61:2587. 73. Gore DM, Leucci MT, Anand V, et al. Combined wavefront-guided transepithelial photorefractive keratectomy and corneal crosslinking for visual rehabilitation in moderate keratoconus. J Cataract Refract Surg. 2018;44:571-80. 74. Kanellopoulos AJ. Ten-year outcomes of progressive keratoconus management with the Athens Protocol (topography-guided partial-refraction PRK combined with CXL). J Refract Surg. 2019;35:478-83. 75. Nattis AS, Rosenberg ED, Donnenfeld ED. One-year visual and astigmatic outcomes of keratoconus patients following sequential crosslinking and topography-guided surface ablation: the TOPOLINK study. J Cataract Refract Surg. 2020;46:507-16. 76. Hersh PS, Issa R, Greenstein SA. Corneal crosslinking and intracorneal ring segments for keratoconus: A randomized study of concurrent versus sequential surgery. J Cataract Refract Surg. 2019;45:830-9. 77. Nguyen N, Gelles JD, Greenstein SA, Hersh PS. Incidence and associations of intracorneal ring segment explantation. J Cataract Refract Surg. 2019;45:153-8. 78. Jacob S, Patel SR, Agarwal A, et al. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34:296-303. 79. van Dijk K, Parker JS, Baydoun L, et al. Bowman layer transplantation: 5-year results. Graefes Arch Clin Exp Ophthalmol. 2018;256:1151-8. 80. Jin H, He M, Liu H, et al. Small-incision femtosecond laser-assisted intracorneal concave lenticule implantation in patients with keratoconus. Cornea. 2019;38:446-53. 81. Kanellopoulos AJ, Vingopoulos F. Combining porcine xenograft intra-corneal ring segments and CXL: a novel technique. Clin Ophthalmol. 2019;13:2521-5. 82. DeNaeyer GSD, Michaud L, Morrison S, et al. Correlation of corneal and scleral topography in cases with ectasias and normal corneas. J Cont Lens Res Sci. 2019;3:e10-e20. 83. Hussoin T, Le HG, Carrasquillo KG, et al. The effect of optic asphericity on visual rehabilitation of corneal ectasia with a prosthetic device. Eye Contact Lens. 2012;38:300-5. 84. Hastings GD, Applegate RA, Nguyen LC, et al. Comparison of wavefront-guided and best conventional scleral lenses after habituation in eyes with corneal ectasia. Optom Vis Sci. 2019;96:238-47. 85. Sabesan R, Johns L, Tomashevskaya O, et al. Wavefront-guided scleral lens prosthetic device for keratoconus. Optom Vis Sci. 2013;90:314-23. |


