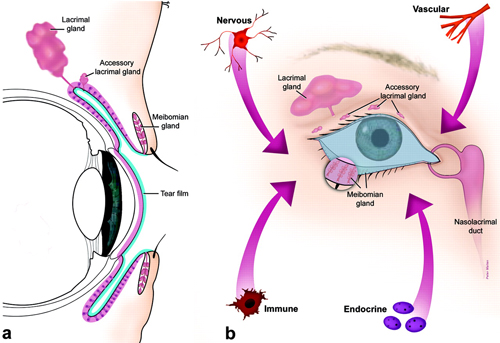
The ocular surface has been described as a system of physical structures working in concert to provide, protect and maintain a smooth refractive surface on the cornea.1 These include the cornea, bulbar and palpebral conjunctivae, lacrimal glands, accessory lacrimal glands and the meibomian glands. It is perhaps tempting to consider each structure in isolation; we tend to think of them as distinct entities with unique anatomical characteristics, functions and vulnerabilities. But a continuous sheet of epithelium spans the ocular surface, with regional specializations along the way (Figure 1), each of which contributes components to the tear film. The functions of each anatomical unit are integrated or linked by innervation and by the endocrine, vascular and immune systems. As such, any dysfunction of the components within this system may alter the refractive surface of the cornea and contribute to signs and symptoms associated with dry eye.1
|
|
|
|
|
Fig. 1. The ocular surface system. (A) Sagittal section showing that the ocular surface epithelium is continuous (pink) with regional specializations on and in the cornea, conjunctiva, lacrimal and accessory lacrimal glands and meibomian gland. Each specialized region of this ocular surface epithelium contributes components of the tear film (blue). (B) Frontal view, which includes the surface and glandular epithelia of the cornea, conjunctiva, lacrimal gland, accessory lacrimal glands and meibomian gland (note enlarged lower lid segment) and their apical (tears) and basal connective tissue matrices, the eye lashes, those components of the eyelids responsible for the blink, and the nasolacrimal duct. The functions of the system’s components are integrated or linked by innervation, and the endocrine, vascular and immune systems. (©Association for Research in Vision and Ophthalmology. From: Gipson IK. The Ocular Surface: The Challenge to Enable and Protect Vision. Invest Ophthalmol Vis Sci 2007;48(10):4391-98.) |
If one considers the ocular surface an interconnected system, it’s logical to investigate whether one condition affecting it contributes to the development of others. For example, it is known that inflammation resulting from allergic eye disease causes surface irregularities to the corneal epithelium and conjunctiva.2 Because the epithelium of the meibomian glands is a continuum of the epithelium of the conjunctiva and cornea, chronic allergic conjunctivitis can affect the epithelium lining the meibomian glands, leading to MGD. From here, inflammation of dysfunctional meibomian glands may ensue, causing posterior blepharitis and further perpetuating the underlying inflammatory response.
Conversely, tear film instability resulting from aqueous and/or lipid deficiencies allows the epithelium of the conjunctiva and cornea to lose its protective tear film, which may result in the development of epithelial irregularities and subsequent symptoms and signs of ocular surface discomfort.2 Loss of the tear film also increases the risk of environmental allergens penetrating the cornea and conjunctiva epithelium, thus increasing the risk for the development of ocular allergy—which creates additional ocular surface inflammation.
Mixed Signals
Given that the ocular surface functions as a system, clinicians should keep in mind that other conditions affecting the ocular surface may not occur as independent disease states either. Most notably, the pathophysiology of MGD is deeply intertwined with that of ocular surface disease. Major landmark publications such as the International Dry Eye Workshop (DEWS) and the International Workshop on Meibomian Gland Dysfunction have provided much-needed consensus definitions of both dry eye and MGD, and each recognized MGD as a major cause of dry eye.2,3
Additionally, they acknowledged dry eye patients likely have elements of both aqueous deficient and evaporative dry eye, and that patients likely have overlapping clinical signs and symptoms consistent with multiple ocular surface conditions.
Mixed presentations are the norm. A recent study of 258 patients used objective testing to classify ocular surface disease into three categories—aqueous tear deficiency (ATD), evaporative dry eye (EDE) and allergic conjunctivitis (AC)—in an attempt to evaluate the prevalence of ocular surface disease sub-types.4 Results showed 86.4% of patients had one or more of the three sub-types, with the most commonly encountered unique or independent condition being AC (41.7%). Combinations of two or more subtypes represented 43.9% of all patients; specifically, 71% of this 43.9% had a combination of allergic conjunctivitis and evaporative dry eye. While this study showed only 13.6% of all patients were unaffected, allergic conjunctivitis was found as either a unique condition or in combination with other subtypes in 73.2% of all patients enrolled in the study.
Another study evaluated morphologic changes of the meibomian glands in patients with and without perennial allergic conjunctivitis, using noncontact meibography.5 Results showed a statistically significant increase in distortion of meibomian gland ducts in patients with perennial allergic conjunctivitis (p=<0.001). A statistically significant difference in meibomian gland score was seen in patients with ductal distortion compared with those without (p=0.0012).
These results provide further evidence that morphologic changes to the meibomian glands are common in patients suffering from allergic conjunctivitis. The authors of this study postulated that eyelid rubbing, which is common among patients suffering from allergic conjunctivitis, could be causing the morphologic changes they observed. This “rubbing” or irritation effect to the MGs was also suggested as an underlying cause for the morphologic changes to the meibomian glands observed in contact lens wearers.5 The researchers used meibography to view and grade meibomian gland loss. A greater loss was observed in contact lens wearers compared to non-contact lens wearers—perhaps epithelial inflammation of the conjunctiva from chronic allergic conjunctivitis and contact lens wear stimulated morphologic changes to the epithelium of the meibomian glands, which may ultimately lead to meibomian gland dysfunction.
As a profession, our understanding of the pathophysiology of meibomian gland dysfunction continues to grow, but neglecting to treat allergic eye disease can result in anatomical changes to the meibomian glands that may ultimately cause symptomatic meibomian gland dysfunction.
When using a symptom-based approach to evaluate the wellness of the ocular surface, several seem to overlap among the various subtypes—namely, ocular irritation, redness, visual discomfort, dryness, burning, soreness and itching. This phenomenon is better understood when one accepts that the ocular surface functions as a system.
Allergic conjunctivitis is often associated with the presence of four classic symptoms: itch, swelling, redness and watery eyes. Itch is considered the primary diagnostic factor for AC; however, a study of 130 previously diagnosed patients with allergic conjunctivitis demonstrated the frequency of burning, soreness and scratchy sensation to be as significant as that of itch in diagnosing.6 Further, the intensity of burning and soreness was more statistically significant than the intensity of itching.
In another study, 689 patients with allergic eye disease and dry eye were surveyed using the Subjective Evaluation of Symptom of Dryness (SESoD) or Frequency of Dryness Score to assess discomfort from itchiness, dryness and redness.7 The results showed that 61.9% of patients with clinically significant itch also had dryness, and 49.9% of patients with clinically significant dryness had itch. The odds of patients experiencing itchy and dry eyes were 2.11 times that of patients without itchy eyes. Similarly, the odds of patients with dry eyes vs. non-dry eyes experiencing itchiness was 2.11.
These results indicate that itch is common in both allergic and dry eye patients; thus, relying solely on symptoms to distinguish between allergic conjunctivitis and dry eye can result in an incorrect diagnosis. Further, it is important to consider that many patients will have more than one type of ocular surface disease—attempting to “shoehorn” a patient into one category may lead to less effective treatment and outcomes.
Bugging Out
Demodex
has recently gained attention with respect to its effects on the ocular surface. Symptoms of Demodex
infestation are similar to those of other ocular surface diseases and include itching, burning, foreign body sensation, blurry vision and crusting and redness of the lid margins.8
Two distinct species of Demodex are known to cause blepharitis. D. folliculorum causes anterior blepharitis when the mite’s claws induce hyperplasia and reactive hyperkeratinization around the base of the lashes, forming cylindrical dandruff.8 D. brevis, meanwhile, causes posterior blepharitis by burrowing itself into the meibomian glands, where it elicits an inflammatory response from the meibomian glands and lid margins. The primary mechanism for this response is thought to result from either delayed hypersensitivity or innate immunity.8
In a study comparing Total Ocular Symptoms Score (TOSS) and other objective clinical signs in Demodex-infested patients and normal patients, results showed a statistically significant difference in mean papillary reaction (p=0.005) and cylindrical dandruff (p<0.0001) between the Demodex group and the normal group.9 Interestingly, however, TOSS—a questionnaire commonly used by allergists to assess allergic eye disease—was not statistically significant between the two groups.
In a second follow-up study, the authors characterized the symptoms associated with D. folliculorum using the Ocular Surface Disease Index (OSDI), Subjective Evaluation of Symptom of Dryness (SESoD), Subjective Evaluation of Frequency of Itch (SEFoI), Subjective Evaluation of Redness (SEoR) and TOSS.10 Results of the OSDI showed 36.1% of patients had symptomatic dry eye (OSDI score ≥13). Clinically significant dryness (SESoD) was found in 23.6% of patients and 20.8% had clinically significant itching (SEFoI), whereas clinically significant itching was found in 27.7% with TOSS. Clinically significant redness (SEoR) was found in 17%. This study also showed clinically significant redness to be more common in patients with higher mite counts.10
With the exception of redness, symptoms do not increase if the infestation is mild or severe, but prolonged infestation of either D. folliculorum or D. brevis can lead to anterior or posterior blepharitis, respectively. This inflammatory response causing blepharitis is not only from the Demodex mite, but also from bacteria that the mite carries, including Staphylococcus and Streptococcus.8 With respect the meibomian glands, prolonged MGD and posterior blepharitis can contribute to hordeolum, chalazion and atrophy of the meibomian glands as seen with advanced stages of MGD.
Beyond the Basics
Dry eye is a multifactorial disease that often requires a multifactorial treatment approach. Understanding the underlying mechanisms for the development of any condition affecting the ocular surface will ultimately allow for more effective treatments. Diagnosing a patient with one subtype of ocular surface disease may lead to ineffective treatment if the patient actually suffers from a mixed form. Relying on a symptom-only approach to assess a dysfunctional ocular surface may also result in an incomplete diagnosis.
Thus, eye care professionals should carefully evaluate all components of the ocular surface system in an attempt to make an all-inclusive or comprehensive diagnosis. Additional work is also needed to develop a questionnaire to increase the sensitivity and specificity of mixed subtypes of ocular surface disease. Recently released research has provided additional evidence to the eye care community to help us better understand the comorbidity of OSD conditions, but additional work is needed to further expand our evidence-based knowledge in this area.
1. Gipson IK. The ocular surface: the challenge to enable and protect vision. The Friendenwald Lecture. Invest Ophthalmol Vis Sci. 2007;10(48):4391-98.
2. Report of the International Dry Eye Workshop (DEWS). Ocul Surf. 2007;5:67-202.
3. Schaumberg DA, Nichols JJ, et al. The int'l workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MDG. Invest Ophthalmol Vis Sci. 2011;52(4):1994-2005.
4. Opitz DL, Kwan JT, et al. Prevalence of allergic conjunctivitis, ocular surface disease subtypes, and mixed diseases. Poster, Association for Research in Vision and Ophthalmology, 2014.
5. Arita R, Itoh K, Maeda S, et al. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea. 2010;29:858-60.
6. Hom MM, Opitz DL, Kwan JT, Bielory L. Severity and frequency of allergic conjunctivitis symptoms. Poster, American Academy of Allergy, Asthma, and Immunology, 2014.
7. Hom MM, Nguyen A, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann Allergy Asthma Immunol. 2012;108:163-6.
8. Lui J, Sheha H, Tseng SC. Pathogenic role of demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10(5):505-10.
9. Hauswirth SG, Hom MM. The relationship of total ocular surface symptoms score (TOSS) to presence of demodex folliculorum. Poster, American Academy of Optometry, 2013.
10. Hauswirth SG, Schachter S, Hom M. Symptoms associated with the presence of demodex folliculorum. Poster, Association for Research in Vision and Ophthalmology, 2014.
11. Simmons PA, Vehige JG, Carlise C, et al. Comparison of dry eye signs in self-described mild and moderate patients. Invest Ophthalmol Vis Sci. 2003:E-Abstract 2448.
12. Hom M, De Land P. Prevalence and severity of symptomatic dry eyes in Hispanics. Optom Vis Sci. 2005;82:206-208.
13. Hom M, De Land P. Self-reported dry eye and diabetic history. Optometry. 2006;77:554-558.
14. Srinivasan S, Simpson TL, Senchyna M, et al. Assessment of ocular surface dryness in postmenopausal females using dry eye questionnaires. Optom Vis Sci. 2008: E-Abstract 80023.
15. Hom M, De Land P. Prevalence of redness and dryness. Optom Vis Sci. 2009: E-Abstract 95581.
16. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615-621.
17. Williams TC, et al. Recognition of allergic conjunctivitis in patients with allergic rhinitis. World Allergy Organ J. 2013;6(1):4.
18. Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-94.


