 There has been plenty said about benzalkonium chloride (BAK) over the years—much attention has been paid to the good, the bad and the ugly when it comes to the preservative which has led to various misconceptions about its use. However, BAK is still the most commonly used preservative in topical multiuse ophthalmic solutions, found in approximately 72% of eye drops.1 Thus, its reputation as the most effective antimicrobial preservative on the market certainly supersedes its negative reviews.
There has been plenty said about benzalkonium chloride (BAK) over the years—much attention has been paid to the good, the bad and the ugly when it comes to the preservative which has led to various misconceptions about its use. However, BAK is still the most commonly used preservative in topical multiuse ophthalmic solutions, found in approximately 72% of eye drops.1 Thus, its reputation as the most effective antimicrobial preservative on the market certainly supersedes its negative reviews.
There have been several misconceptions about the preservative, but perhaps one of the more troubling concerns stems from the safety of BAK use with contact lenses and the appropriate waiting time between dosage and lens wear. In this column we will take a look at the background and package insert instructions to better understand the use of products containing BAK.
Dosage Allowance
While detractors of the use of BAK claim corneal toxicity as a major concern, the true culprit is dosing. Of course, when used chronically, and to extremes, BAK may damage the ocular surface.2 However, BAK does not appear to have adverse effects at concentrations used clinically (0.004% to 0.02%) unless the frequency of use exceeds four to six times daily.3 Researchers from Schepens Eye Research Institute found that when rabbits were given two drops of 0.02% BAK every three minutes for one hour, cytotoxic morphological changes were observed in the corneal epithelium.3 However, in reality, this excessive amount of dosing would never be administered on a human patient.
Typically, a 10 to 15 minute wait to reinsert a contact lens is required after administering a drop containing BAK. However, some recent studies have proven that the wait period is not only outdated, but also unnecessary. Mike Christensen, PhD, and colleagues examined the absorption of active ingredients into the lens after the instillation of the short-action, anti-allergic combination therapy Naphcon-A (naphazoline/pheniramine, Alcon) containing 0.01% BAK. After instillation of Naphcon-A, the patient was instructed to wait five minutes before inserting the lens into the eye.4 The lenses were then collected after one week of use and results showed that removing the lenses for five minutes was, in terms of absorption of the solution, equivalent to not wearing the lenses at all. Essentially, the amount of BAK that could be extracted from the lenses was below the limits of detection.
In another study on BAK content in contact lenses, Wakako Iwasaki, M.D., and colleagues examined the mast cell stabilizer disodium cromoglycate, which has a BAK concentration of 100µg/mL, and administered the solution four times daily for one month to 10 patients using extended wear contact lenses.5 The BAK content was found to be below the level of detection (10ppm) in the lenses, which were all assayable for BAK.5
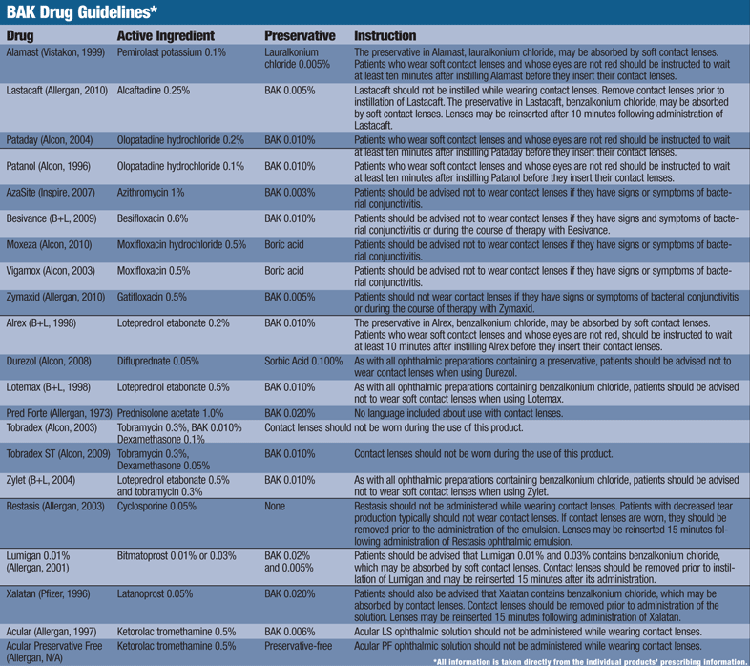
Waiting Time
Further investigation into the instructions of today’s leading ophthalmic solutions reveals an array of inconsistencies with BAK guidelines. Typically, ophthalmic antihistamines recommend a 10-minute wait period before putting the contact lens back into the eye; glaucoma medications require a 15-minute wait, while with NSAIDS and most anti-inflammatories contact lens use is not advised.6-12 However, there does not seem to be a truly consistent thread for these guidelines, whether it is because of the active ingredient, BAK percentage or type of preservative.
For example, the package insert for the steroidal anti-inflammatories Zylet (loteprednol etabonate 0.5%/tobramycin 0.3%, Bausch + Lomb) and Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb) state that all drops containing benzalkonium chloride should not be used with contact lenses. However, Alrex (loteprednol etabonate 0.2%, Bausch + Lomb), a steroidal anti-inflammatory drug with a lower active concentration of loteprednol etabonate than Lotemax but with the same amount of BAK (0.01%), is safe to use with contact lenses after waiting 10 minutes.13-15
Similarly, the 15-minute wait suggested for glaucoma medications is refuted by package inserts for Xalatan (latanoprost 0.05%, Pfizer) and Lumigan (bimatoprost 0.01% or 0.03%, Allergan), which specifically state that BAK may be absorbed by soft contact lenses without the 15-minute wait.9,10 Even more confusing is the package insert for Restasis (cyclosporine 0.05%, Allergan), which does not contain BAK, yet reads “if contact lenses are worn, they should be removed prior to the administration of the emulsion. Lenses may be reinserted 15 minutes following administration of Restasis ophthalmic emulsion.”16 Overall, it is difficult to predict what the contact lens guideline will be, and whether BAK is even a contributing factor.
All in all, the consensus for BAK and its correlation to contact lenses may need to be reconsidered—partly due to a better understanding of the preservative, its absorption into lenses and the vast inconsistencies concerning the package guidelines for BAK use. Along those lines, the future of contact lenses is evolving as new drug/device combinations are developing in innovative ways. Ultimately, BAK is a preservative that, with normal dosing, is vastly effective and should continue to be used in ophthalmic preparations.
1. PDR for Ophthalmic Medicines.
2. Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008 Nov;86(7):716-26.
3. Berdy GJ, Abelson MB, Smith LM, George MA. Preservative-free artificial tear preparations. Assessment of corneal epithelial toxic effects. Arch Ophthalmol. 1992 Apr;110(4):528-32.
4. Christensen MT, Barry JR, Turner FD. Five-minute removal of soft lenses prevents most absorption of a topical ophthalmic solution. Clao J. 1998 Oct;24(4):227-31.
5. Iwasaki W, Kosaka Y, Momose T, Yasuda T. Absorption of topical disodium cromoglycate and its preservatives by soft contact lenses. Clao J. 1988 Jul-Sep;14(3):155-8.
6. Lastacaft. Allergan. Package Insert. 2010.
7. Patanol. Alcon. Package Insert. 1996.
8. Pataday. Alcon. Package Insert. 2004.
9. Xalatan. Pfizer. Package Insert. 1996.
10. Lumigan. Allergan. Package Insert. 2001.
11. Restasis. Allergan. Package Insert. 2003.
12. Allergan. Pred Forte. Package Insert. 2004.
13. Zylet. Bausch + Lomb. Package Insert. 2004.
14. Lotemax. Bausch + Lomb. Package Insert. 1998.
15. Alrex. Bausch + Lomb. Package Insert. 1998.
16. Restasis. Allergan. Package Insert. 2003.


