Contact lens care is a complex topic. With a recent surge in contact lens brand, modality and solution availability, there are important considerations at play in determining an optimal contact lens care regimen for patients to enhance comfort, maintain ocular surface health and reduce contact lens dropout. Despite the increasing trend toward fitting soft daily disposable contact lenses, there are still patients continuing with biweekly and monthly soft lenses, as well as those wearing custom soft lenses for aphakia with a quarterly replacement schedule.
Annual modalities include hybrid lenses and rigid gas permeable (GP) lenses such as corneal and scleral GPs. Different lenses with different replacement schedules necessitate an understanding of the associated care regimens to reduce patient discomfort, lens dropout and adverse events such as microbial keratitis (MK) and inflammatory events.
Soft Contact Lens Solutions
With daily disposable contact lens use on the rise, we have even more of a duty to our patients who rely on biweekly, monthly and quarterly soft lens modalities. For instance, pediatric patients with congenital pathologies and patients with high refractive powers outside the range of dailies are often fitted in monthly to quarterly soft lenses for vision rehabilitation. This increases the importance of prioritizing lens disinfection to prevent incidences of MK and ocular surface compromise.
Multipurpose solution (MPS). This option is excellent for its ease and convenience of use. However, there has been an ongoing debate on the biocompatibility of soft contact lens material and these solutions and their impact on corneal health. Corneal staining grids across multiple lens brands and solution types highlight the solution-induced corneal staining after two to four hours of lens wear.1,2 Initially, it was believed that solutions with PHMB preservatives were the cause of damaged epithelial cells resulting in corneal staining. However, further research shows that MPS can cause alterations to the cell membrane in its uptake of fluorescein without causing cell death.3,4 Another study noted that the surfactants, not the preservatives, impact the active transport of fluorescein across the epithelial membrane.5
MPS is a one-step system that is effective in cleaning, disinfecting and storing contact lenses. The different formulations play a significant role in improving patient comfort, minimizing risk of ocular infection and optimizing longer wear time with wetting agents. The presence of buffers, surfactants and chelators optimizes compatibility of the solution with the tear film to allow for homeostasis of the ocular surface.6 Some products have received FDA clearance for rinse-only instead of the traditional rub and rinse technique; however, studies have shown that rinse-only MPS is ineffective in removing protein deposits from the lens surface, leaving up to 40% behind.7-10 Presence of denatured proteins on the lens surface can result in clinically significant concerns such as contact lens-induced papillary conjunctivitis.9 The newer generation of MPS contains dual disinfectants, which boast excellent biocompatibility and a robust disinfection and cleaning capability.11
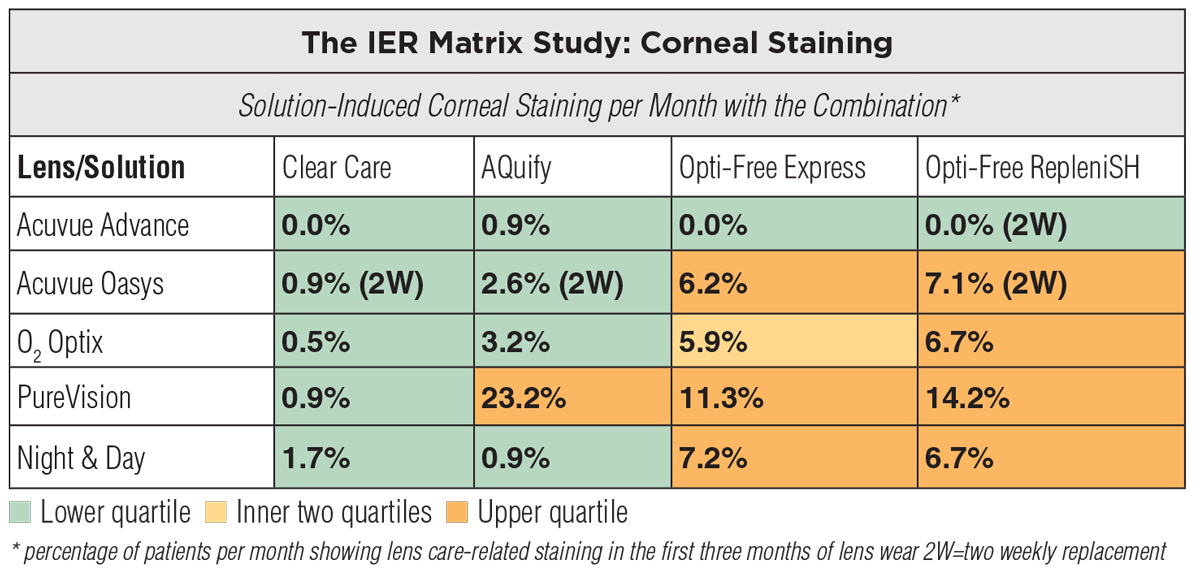 |
Corneal staining occurs as a result of an incompatibility between the lens solution and material. Click image to enlarge. |
Hydrogen peroxide. This other popular system is a great choice for soft, hybrid and GP lenses. The stabilized 3% hydrogen peroxide system is protective against bacteria, viruses, fungi and protozoan through oxidation.12-14 Unlike some MPS, it is effective and can penetrate several microbial biofilms.15
The main concerns with this disinfection system are twofold: chemical burn of the ocular surface in cases of incomplete neutralization of the peroxide and potential contamination of lenses that are bathed in neutralized, unpreserved solution following completion of the neutralization process. Therefore, it is important to educate patients about the proper way to use the solution and discuss re-disinfecting the lenses if they are left soaking in the solution for longer than one to two days.16 This system is perfect for patients with ocular allergies and sensitivities as they might develop a delayed hypersensitivity response to preservatives and other agents in MPS.
Hybrid Contact Lens Solutions
The unique category of lenses that have a rigid GP center and a surrounding soft skirt are known as hybrids. These lenses are compatible with hydrogen peroxide or MPS approved for soft contact lenses. For patients with concerns of lens deposits, soft lens daily cleaners can be added to the regimen for thorough lens disinfection. Caution is needed when using abrasive or alcohol-based cleaners as they can strip the hybrid lens surface coating.
GP Contact Lens Solutions
Similar to soft contact lenses, GP lenses require a good disinfecting and conditioning regimen to improve their longevity, provide comfort and minimize the risk of infection. Since these lenses have an annual replacement schedule, it becomes even more important to thoroughly educate patients on lens hygiene and care.
Recommendations For Contact Lens CareKeep these tips and tricks in mind when performing your care routine:
|
One-step MPS. Similar to soft lenses, solutions for GP lenses have also headed toward multipurpose cleaners. All-in-one products that allow for cleaning, disinfecting, conditioning and rinsing provide ease and convenience for patients. One-step MPS usually contains preservatives, viscosity and cushioning agents to improve surface wettability and a low concentration of surfactants.10,16-18
These products are especially helpful when working with children who require GP lenses for ocular pathologies or orthokeratology lenses. Since MPS is generally non-abrasive in nature, it is compatible with lens coatings on corneal and scleral GPs such as plasma treatment and polyethylene glycol (PEG) surface coating.10 However, caution should be taken when working with patients with a pre-existing history of ocular allergies and sensitivities as they may experience adverse effects with the preservatives in MPS.
Multistep cleaning system. There are a range of multistep products. Two-step GP lens cleaning systems involve the use of a cleaning solution and a disinfecting/conditioning agent. Usually the cleaning solution is an abrasive, concentrated surfactant solution containing silica gel beads that maximize cleaning but are incompatible with plasma and PEG surface treatments. Some manufactures offer three-step systems that involve a cleaning/disinfecting/soaking solution containing benzyl alcohol, an extra-strength cleaner and a wetting solution. The soaking solution needs to be rinsed off and the lenses need to be re-wetted with the wetting solution prior to use. The presence of benzyl alcohol makes the cleaner effective against lipid deposits.10,16-18
Multistep systems are an excellent choice for patients with ocular surface disease that results in heavy protein and lipid deposits on the lens surface. In addition, the use of extra-strength cleaners may prevent the long-term deposit buildup on the lens surface that can reduce the lifespan of the lens and may require more frequent replacement.
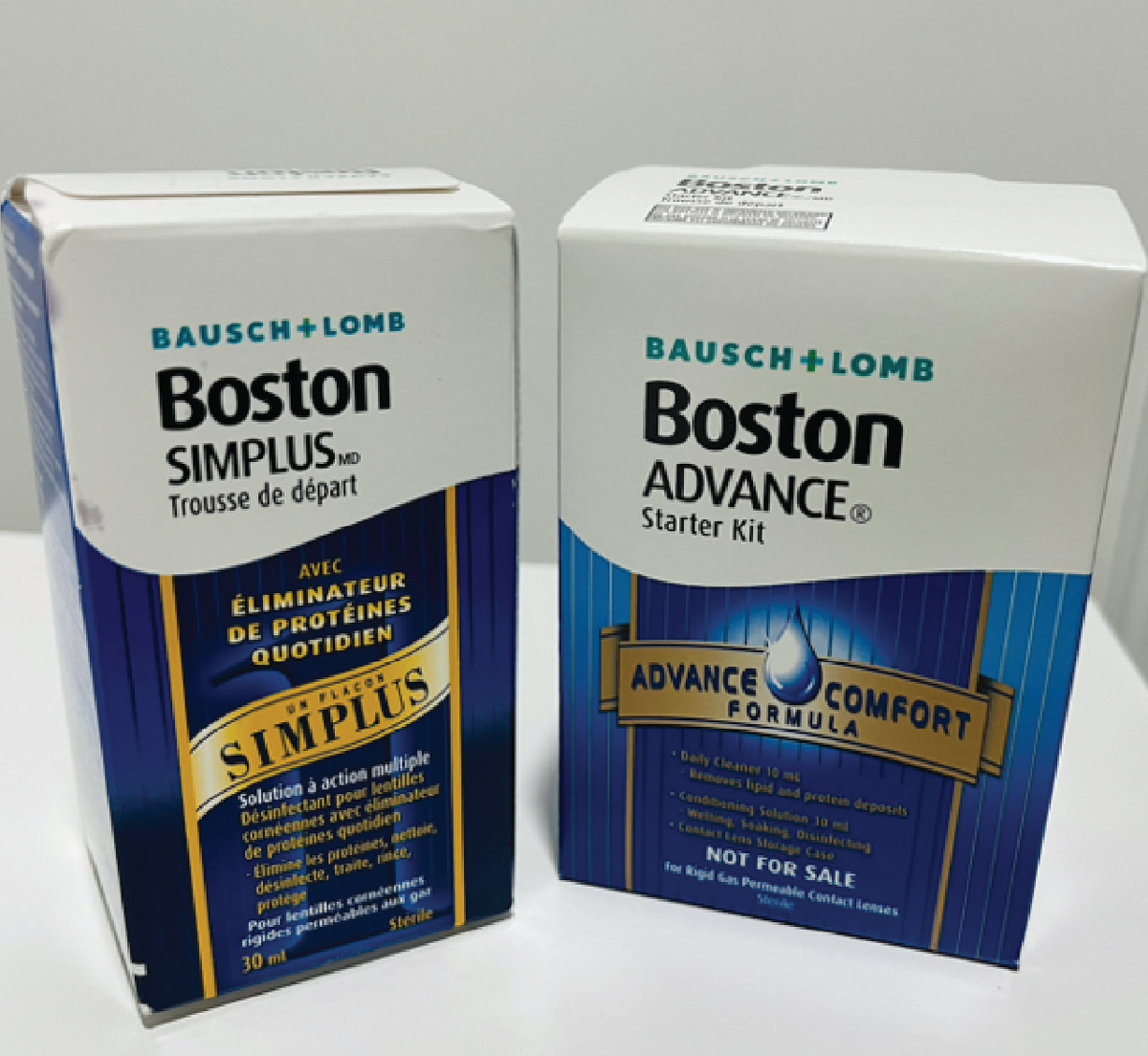 |
For GP lenses, Boston Simplus is a one-step multipurpose system, whereas Boston Advance is a multistep cleaning system. Click image to enlarge. |
Protein cleaners. Contact lens materials have changed dramatically over the past few years. The addition of silicone-containing monomers resulted in increased oxygen permeability of the lens; however, it also increased the material’s propensity for protein deposits.19 With the newer fluorosilicone materials, there is improved oxygen permeability with the added advantage of the material’s ability to resist mucin and other deposits.19 However, patients with ocular surface disease have a tear film imbalance that can result in surface buildup with long-term wear.
Daily protein removers and treatments like a 30-minute progent procedure are effective in removing lens deposits. Progent treatment can be performed bi-weekly at home for clearing protein buildup and disinfecting against viruses, Acanthamoeba and other microorganisms.
Digital Contact Lens Rubbing
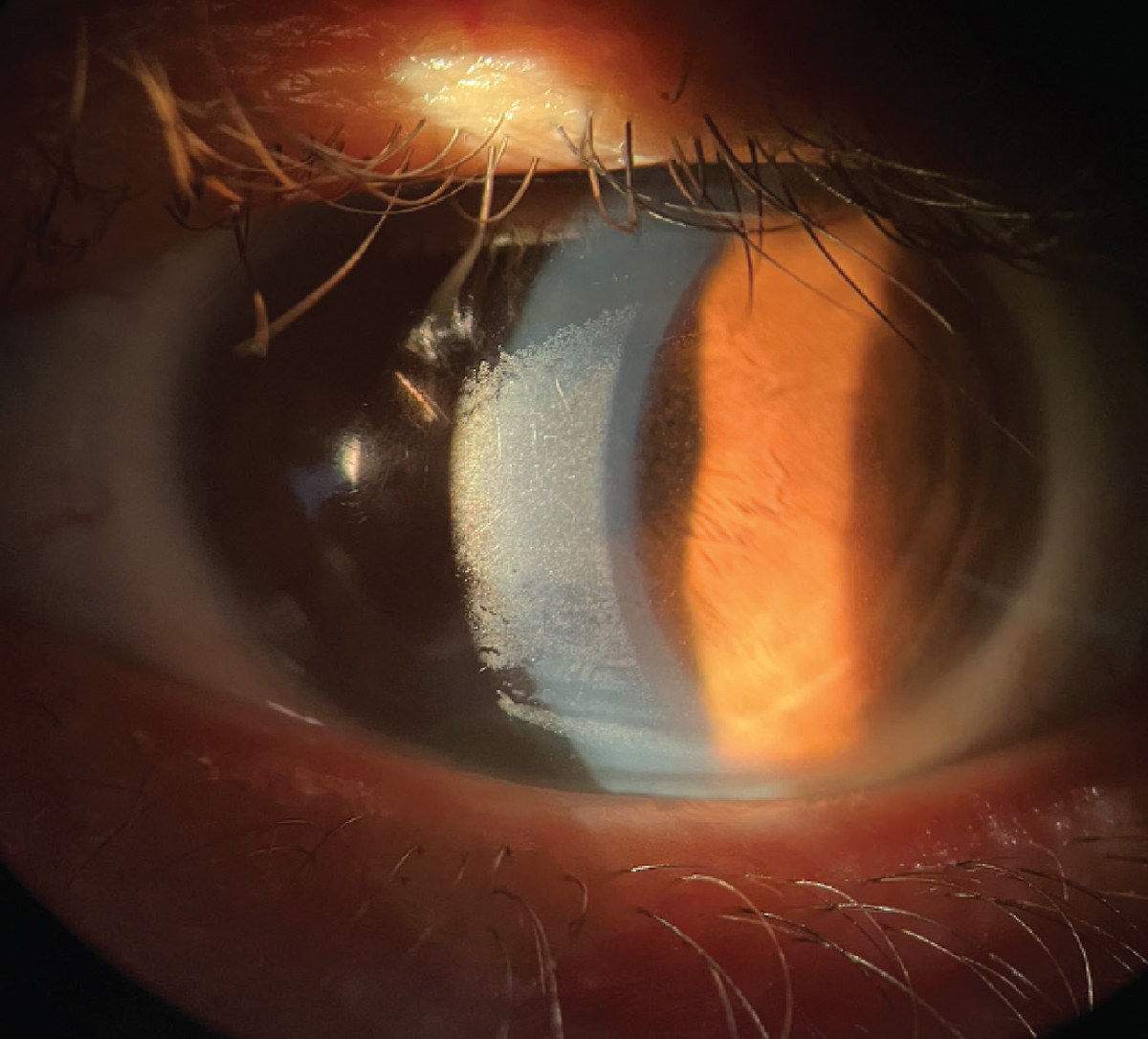 |
|
Build-up on a scleral lens surface can form from a poor tear film. Click image to enlarge. |
This method can remove dirt, debris and cosmetics that might adhere to the lens surface (both soft and GP lenses). There is strong evidence that digital rubbing of lenses can help with removing biofilms and preventing Acanthamoeba, Pseudomonas aeruginosa, Staphylococcus aureus, Fusarium solani and Candida albicans.20,21 One study examined the amoebicidal activity of disinfecting agents containing hydrogen peroxide, chlorhexidine, thimerosal, chlorhexidine, thimerosal-polyquaternium and polyaminopropyl biguanide-poloxamine.22 The researchers noted that the cleaning agents were ineffective in removing all Acanthamoeba cysts during the 17-hour experiment and reinforced the need for mechanical rubbing of the lenses.22 Rubbing the lens surface for approximately 10 seconds on each side is usually recommended for thorough cleaning.
Contact Lens Case Replacement
Contact lens cases contain microorganisms such as micrococcaceae Pseudomonadaceae, Enterobacteriaceae and amoeba. Improper handling of contact lenses can result in contamination and corneal infection.23 The incidence of microbial burden within storage cases ranges from 24% to 81%.24
Once a biofilm forms from coalesced microbial colonies, it creates resistance to lens care products. Studies have shown that the polyquaternium-preserved solution is effective in reducing transfer of bacterial microbes from a lens case to a silicone hydrogel lens that was soaked in the case.24,25 Frequent lens case replacement can prevent microbial activity and reduce the risk of MK.6
Takeaways
Contact lens hygiene and care is a crucial part of the contact lens fitting process. Not all solutions are made the same way, and patients can develop sensitivities or reactions to certain chemicals in them. Working with patients to develop a customized cleaning regimen to fit their personal needs can make a world of difference. Education on solution options, digital rubbing and replacement schedules helps build a strong foundation for good habits, patient compliance and minimized risks.
Dr. Bedi completed a residency in cornea and contact lenses at the Southern California College of Optometry at Marshall B. Ketchum University after graduating from the Illinois College of Optometry. She focused on specialty contact lens fitting for corneal pathologies, aphakia and prosthetics. She is a fellow of both the Scleral Lens Society and the American Academy of Optometry.
1. Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses and multipurpose solution combinations. Optometry. 2008;79(8):444-54. 2. Carnt N, Willcox MD, Evans V, et al. Corneal staining: the IER Matrix study. Contact Lens Spectrum. September 1, 2007. 3. Bandamwar KL, Papas EB, Garrett Q. Fluorescein staining and physiological state of corneal epithelial cells. Cont Lens Anterior Eye. 2014;37(3):213-23. 4. Bakkar MM, Hardaker L, March P, et al. The cellular basis for biocide-induced fluorescein hyperfluorescence in mammalian cell culture. PLoS ONE. 2014;9(1):e84427. 5. Khan TF, Price BL, Morgan PB, et al. Cellular fluorescein hyperfluorescence is dynamin-dependent and increased by Tetronic 1107 treatment. Int J Biochem Cell Biol. 2018;101:54-63. 6. Bright FV, Burke SE, Chalmers RL, et al. Contemporary research in contact lens care. Cont Lens Anterior Eye. 2013;36(S1):S22-7. 7. Mok KH, Cheung RWL, Wong BKH, et al. Effectiveness of no-rub contact lens cleaning on protein removal: a pilot study. Optom Vis Sci. 2004;81(6):468-70. 8. Pucker AD, Nichols JJ. Impact of a rinse step on protein removal from silicone hydrogel contact lenses. Optom Vis Sci. 2009;86(8):943-7. 9. Eiden B. Does one contact lens solution fit all? Review of Cornea & Contact Lenses. May 18, 2011. 10. Quinn TG. Making sense of contact lens care. Contact Lens Spectrum. November 1, 2017. 11 Reindel W, Merchea MM, Rah MJ, Zhang L. Meta-analysis of the ocular biocompatibility of a new multipurpose lens care system. Clin Ophthalmol. 2013;7:2051-6. 12. Johnston SP, Sriram R, Qvarnstrom Y, et al. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J Clin Microbiol. 2009;47:2040-5. 13. Hughes R, Kilvington S. Comparison of hydrogen peroxide contact lens disinfection systems and solutions against Acanthamoeba polyphaga. Antimicrob Agents Chemother. 2001;45:2038-43. 14. Lever AM, Sutton SVW. Antimicrobial effects of hydrogen peroxide as an antiseptic and disinfectant. In: Handbook of Disinfectants and Antiseptics. Ascenzi JM, ed. CRC Press, 1995. 15. Linley E, Denyer S, McDonnell G, et al. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67:1589-96. 16. Ward M. Corneal and scleral GP contact lens care. Contact Lens Spectrum. November 1, 2018. 17. Benoit DP. Proper care of modern GP lenses. Contact Lens Spectrum. May 1, 2015. 18. Watanabe RK, Rah MJ. Preventative contact lens care: Part III. Contact Lens Spectrum. August 1, 2001. 19. Young, R. The evolution of contact lens materials. Contact Lens Spectrum. November 1, 2017. 20. Zhu H, Bandara M, Kumar A, et al. Contribution of regimen steps to efficacy of multipurpose solutions used to disinfect silicone hydrogel contact lenses. XVIII International Congress of Eye Research, Beijing, September 24-29, 2008. 21. Butcko V, McMahon TT, Joslin CE, Jones L. Microbial keratitis and the role of rub and rinsing. Eye Contact Lens. 2007;33(6):421-3. 22. Cancrini G, Iori A, Mancino R. Acanthamoeba adherence to contact lenses, removal by rinsing procedures, and survival to some ophthalmic products. Parassitologia. 1998;40:275-8. 23. Pens CJ, Costa MD, Fadanelli C, et al. Acanthamoeba spp. and bacterial contamination in contact lens storage cases and the relationship to user profiles. Parasitol Res. 2008;103:1241-5. 24. Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens. 2010;36(2):116-29. 25. Vermeltfoort PB, Hooymans JM, Busscher HJ, et al. Bacterial transmission from lens storage cases to contact lenses-effects of lens care solutions and silver impregnation of cases. J Biomed Mater Res B Appl Biomater. 2008;87:237-43. |


