Defining the Terms
Biocompatibility is traditionally defined as a medical device free of toxic or injurious effects on biological systems, or the extent to which a foreign material fails to elicit an immune or other response in the recipient. Eye care practitioners may want to take this a step further to include the concept of mimicking a part of the body (e.g., the ocular surface), where a device such as a contact lens is placed. That is to say, a biocompatible product would have some property, whether inherent in the contact lens polymer or added to the lens after polymerization, that is similar to the cornea or components of the tear film.
Omafilcon A
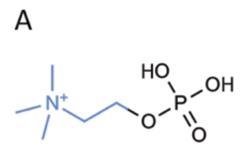
1a. Chemical structure of phosphorylcholine.
While many lenses might be described as biocompatible, omafilcon A (Proclear, CooperVision) was one of the first polymers touted to mimic the ocular surface. Omafilcon A is a 62% water content material composed of 2-hydroxyethylmethacrylate (HEMA) and 2-methacryloyloxyethyl phosphorylcholine (PC) polymers crosslinked with ethyleneglycol dimethacrylate. PC is similar to phospholipids (e.g., phosphatidylcholine), which are molecules found naturally in human cell membranes (figures 1a and 1b).
Due to their zwitterionic nature (e.g., they contain both positive and negative charges), these molecules can bond hydrogen with water. Therefore, they attract and surround themselves with water molecules. This property is why daily disposable manufacturers claim that omafilcon A lenses stay “moist and comfortable, even after 12 hours of wear.”
According to the manufacturer, omafilcon A lenses are the only contact lenses cleared by the FDA to “provide improved comfort for contact lens wearers who experience mild discomfort or symptoms relating to dryness during lens wear.”1 Studies have shown a reduced likelihood of omafilcon A lenses to dehydrate on-eye.2-5

1b. Chemical structure of phosphatidylcholine.
Despite increased tear film evaporation rates and decreased tear film break-up times (TFBUTs), omafilcon A lenses have been shown to be relatively sparing of the pre-lens tear film lipid layer structure.6 Omafilcon A lenses show improved symptoms of dryness and discomfort––as well as improved fluorescein and lissamine green staining––compared to habitual lenses in some studies, (although others conflict).3,7-9
Delefilcon A
A lens material containing phosphatidylcholine is the newest technology on the market. The delefilcon A lens (Alcon) is a silicone-containing hydrogel described as possessing a water gradient—from 33% water at core to >80% water at surface, which is only 6µm thick. The manufacturer claims that the 80% water content closely mimics that of the cornea. In addition, this lens is packaged in phosphate-buffered saline solution containing approximately 0.3% of polymeric wetting agents—copolymers of polyamidoamine and poly(acrylamide-acrylic acid). The material is sold for single use daily disposable lens wear.
The published literature on the new delefilcon A material is limited, but research is beginning to show an association between its lubricity profile and subjective reports of comfort.10 It is known to have a low contact angle hysteresis, which implies better surface wettability (at least in the laboratory). Other physicochemical and biochemical factors beyond wettability also influence ocular response to lens wear and will require study.11 It will be interesting to see if the reports of good clinical performance of the omafilcon A lens, which contains phosphorylcholine—chemically similar to the phosphatidylcholine in delefilcon A—are replicated for this lens type.
Nelfilcon A
Another lens material described as biocompatible is nelfilcon A (Alcon), a polymer of polyvinyl alcohol partially acetalized with N-formylmethyl acrylamide and with a 69% water content. The packaging contains the moisturizing agents hydroxypropyl methylcellulose (HPMC), polyethylene glycol (PEG) and polyvinyl alcohol (PVA). The manufacturer calls PVA (figure 2) a comfort enhancer that gives the lens “outstanding” hydrophilic properties, such as a very low contact angle (which provides better moisture coverage). PVA is also described an agent that helps to stabilize the tear film and that works in synergy with the other agents to continuously lubricate the lens for improved comfort.
HPMC is claimed to optimize the viscosity of the packaging solution to provide superior comfort upon insertion. The manufacturer states that the eye’s natural blinking action releases PVA from the lens, and it and the other moisturizing agents are gradually released over a 20-hour period. Furthermore, this release is associated with “built-in, single-day compliance.”
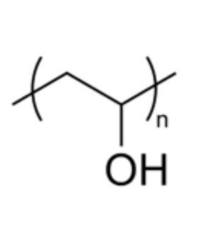

2, 3. Left: Chemical structure of PVA (“n” indicates repeating units of this basic structure to form the polymer).Right: Chemical structure of N-vinyl-pyrrolidone.
Prior to the release of this lens, PVA was used in ocular drops and shown to improve TFBUT. If used at a concentration of 1.4% (w/v) PVA improved TFBUT by 1.89 times, and if used at 10% (w/V) TFBUT was improved by 7.16 times.12 Nelfilcon A lenses with Aqua Release (extra PVA eluting from lenses) provided increased comfortable wearing time and ocular comfort compared to other daily disposable lenses.13,14 Peterson and associates showed a slight improvement in non-invasive break-up time (NIBUT) of tears on the lens surface for the nelfilcon A containing Aqua Release compared to original nelfilcon A lenses, although this did not reach significance.13 However, another study indicated a significant enhancement in NIBUT with the nelfilcon A Aqua Release lenses.15
Nesofilcon A
Nesofilcon A (Bausch + Lomb), a hydrophilic copolymer of 2-hydroxyethyl methacrylate and N-vinyl pyrrolidone, is sold as a daily disposable lens. This lens is marketed as one that “matches the moisture level of your eyes to bring you comfortable vision all day long” and that the concept was “inspired by the biology of your eyes.” This “bioinspired” material exhibits an outer lens surface that is designed to mimic the tear film’s lipid layer in an effort to prevent dehydration and maintain consistent optics. The manufacturer points to its water content of 78% (the same as that of the cornea) as a factor that will “support incredible comfort.” The lipid layer mimicry is provided by the N-vinyl pyrrolidone moiety (figure 3).
The vinyl moiety has the ability to interact with lipids, such as phospholipids found in the tear film layer and plasma membranes.
As yet there are no peer- reviewed studies on the biocompatibility of nesofilcon A lenses. However, N-vinyl pyrrolidone (NVP) and its polymeric form polyvinyl pyrrolidone (PVP) have been used in many different lenses over the years, such as senofilcon A (which uses PVP) or vifilcon A (containing NVP). However, whether the improvements in symptoms or clinical performance with senofilcon A lenses is due to the presence of the vinyl pyrrolidone or other factors such as lens design is not known.16 Recent evidence suggests that the presence of PVP in narafilcon A lenses is not associated with improved clinical performance in terms of dryness and corneal staining.17
Contact lenses generally are biocompatible with the ocular surface, in the sense that they are not toxic and are generally well tolerated by the ocular tissue. The development of materials that are attempting to mimic specific aspects of the ocular surface—be that the water content of the cornea or a structuring of the tear film or the lipid layer of the tear film—is exciting. It seems the days are gone when it was thought that to be biocompatible, the lens should have no interactions with the eye.
It will be interesting to see whether the claims of lenses with surface water content similar to the cornea translate into improved clinical performance and biocompatibility with the cornea. Past experience with high water HEMA-based lenses showed equivocal results for dryness symptoms during wear, and dryness symptoms in relation to lens dehydration.3,18,19 Perhaps the new generation of high water content lenses, or high water content lens surfaces, do not dehydrate as much or as rapidly on-eye? I eagerly await results of clinical trials with these new lenses.
Professor Willcox is a fellow of the BCLA and AAO, and president of the ISCLR. He has specialized in ocular microbiology, contact lenses, tear film biochemistry and corneal immunology. His research has examined the interactions of the normal microbiota of the eye with contact lenses and the effect of contact lenses on the tear film.
1.
http://coopervision.com/contact-lenses/proclear-comfortable-contacts. Accessed 16th March 2013.
2. Hall B, Jones S, Young G, Coleman S. The on-eye dehydration of proclear compatibles lenses. CLAO. 1999 Oct;25(4):233-7.
3. Fonn D, Situ P, Simpson T. Hydrogel lens dehydration and subjective comfort and dryness ratings in symptomatic and asymptomatic contact lens wearers. Optom Vis Sci. 1999 Oct;76(10):700-4.
4. Young G, Bowers R, Hall B, Port M. Clinical comparison of Omafilcon A with four control materials. CLAO. 1997 Oct;23(4):249-58.
5. Young G, Bowers R, Hall B, Port M. Six month clinical evaluation of a biomimetic hydrogel contact lens. CLAO. 1997 Oct;23(4):226-36.
6. Thai LC, Tomlinson A, Doane MG. Effect of contact lens materials on tear physiology. Optom Vis Sci. 2004 Mar;81(3):194-204.
7. Riley C, Chalmers RL, Pence N. The impact of lens choice in the relief of contact lens related symptoms and ocular surface findings. Cont Lens Anterior Eye. 2005 Mar;28(1):13-9.
8. Lemp MA, Caffery B, Lebow K, et al. Omafilcon A (Proclear) soft contact lenses in a dry eye population. CLAO. 1999 Jan;25(1):40-7.
9. Fonn D, Dumbleton K. Dryness and discomfort with silicone hydrogel contact lenses. Eye Contact Lens. 2003 Jan;29(1 Suppl):S101-4.
10. Kern JR, Rappon J, Bauman E, Vaughn B. Assessment of the relationship between contact lens coefficient of friction and subject lens comfort. ARVO 2013 abstract B0131.
11. Tighe BJ. A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye Contact Lens. 2013 Jan;39(1):4-12.
12. Bartlett JD, Jaanus SD. Clinical ocular pharmacology. 5th ed. St. Louis, Mo.: Butterworth-Heinemann/Elsevier; 2008:xvi, 793.
13. Peterson RC, Wolffsohn JS, Nick J, et al. Clinical performance of daily disposable soft contact lenses using sustained release technology. Cont Lens Anterior Eye. 2006 Jul;29(3):127-34.
14. Wolffsohn JS, Hunt OA, Chowdhury A. Objective clinical performance of ‘comfort-enhanced’ daily disposable soft contact lenses. Contact Lens Anterior Eye. 2010;33:88-92.
15. Golebiowski B, Phillips B, Willcox M. Improved non-invasive tear break-up time with nelfilcon A daily disposable soft contact lenses with added PVA. Presented at the 30th conference of the British Contact Lens Association; 2006.
16. Spyridon M, Hickson-Curran S, Hunt C, Young G. Eye sensitivity in soft contact lens wearers. Optom Vis Sci. 2012 Dec;89(12):1682-90.
17. Diec J, Lazon de la Jara P, Willcox M, Holden BA. The clinical performance of lenses disposed of daily can vary considerably. Eye Contact Lens. 2012 Sep;38(5):313-8.
18. Pritchard N, Fonn D. Dehydration, lens movement and dryness ratings of hydrogel contact lenses. Ophthalmic Physiol Optics. 1995 Jul;15(4):281-6.
19. Efron N, Brennan NA, Bruce AS, et al. Dehydration of hydrogel lenses under normal wearing conditions. CLAO. 1987 May/Jun;13(3):152-6.


