The Evolution of Care Systems
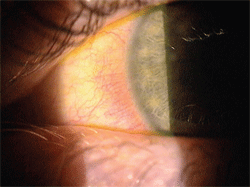 |
| 1. Limbal hyperemia present in a patient wearing a high myopic traditional hydrogel contact lens. |
But, along with the availability of these new materials came an industry demand for better contact lens care systems—ones that were better suited to clean and moisturize the surfaces of these increasingly complex materials. Less than a decade ago, we witnessed the introduction of a new generation of contact lens care systems; these solutions––created in the era of silicone hydrogel lenses––attempted to meet the demands of the new materials that were being increasingly utilized by practitioners.
The complexity in the development of these new systems came to light in 2006 and 2007 when two contact lens solutions were recalled. The first solution was recalled in 2006 after an increase of Fusarium keratitis cases among contact lens users.7,8 In 2007, a second solution was recalled after it was connected to an increase of Acanthamoeba keratitis amongst lens wearers.9 These two events highlighted the delicate balance that needs to exist in order to preserve the patients’ comfort while providing adequate disinfection efficacy.
Reinforce Proper Care
With all of the variability and complexity that exists with contact lens solutions and their influence on a patient’s wearing experience, it is critical for practitioners to better understand what choices our patients are making on how to care for their lenses. We’ve seen first-hand the sequelae of patients who stray from our recommendations when they leave the office and select contact lens care systems other than what was recommended. The additional time that it takes to troubleshoot the subsequent complaints that these patients have is significant. Until we have all the information regarding the care of the lenses, we will have a difficult time deducing the ultimate cause of the complaints our patients may have (figure 2).
 |
| 2. Poor patient compliance demonstrated by the condition of the contact lens case. |
This is a critical first step in patient education. It holds patients accountable on their lens care regimen, and does so in anon-confrontational manner. It also creates an environment where the clinician can have an open dialogue on the proper care habits, including choice of solution.
From a practitioner’s perspective, the question of effective lens care would be a far more simplified process if contact lens solutions were a prescribed product, such as the contact lenses we fit; we would know then that our patients are using exactly what we prescribed. Following that logic, I recommend writing out the type of contact lens solution you have recommended on a prescription pad for your patients to take with them as they leave your office. This may help reinforce verbal instructions, particularly for those patients who tend not to follow your recommendation.
You should always back up your recommendations by sharing your reasoning. We find that patients who have experienced an adverse event with their contact lenses are usually, although certainly not always, more compliant with our instructions. However, for patients who have not experienced an adverse event, sometimes it is difficult for them to appreciate potential adverse events that they may encounter by continuing with non-compliant contact lens care. In those instances, we find that open communication regarding the comfort of lens wear can help maximize the chances of long-term compliance.
Prioritize Compliance
In 2009, Kathy Dumbleton, M.Sc., and colleagues published an interesting study on patient compliance where they asked compliant and non-compliant lens wearers several questions about the wear and care of their contact lenses.10 Each group answered the questions as expected, and the responses varied significantly and for the most part, were somewhat predictable for each group. Interestingly, however, one statement resonated equally with both compliant and non-compliant wearers: how comfort affects the way they wear their lenses.
Eighty-five percent of compliant wearers and 83% of non-complaint wearers agreed with the statement that uncomfortable lenses made them more likely to follow the recommended replacement schedule.10 This finding demonstrated the importance that comfort has on both groups of lens wearers; knowing this, we can leverage comfort as an important variable when describing proper lens wear and care with our patients.
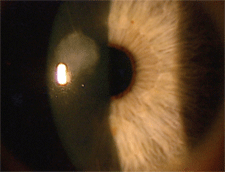 |
| 3. Central stromal scar secondary to a history of a corneal ulcer one year prior. |
The above outlined process helps us do a number of things. First, it helps decommoditize the services and products that we provide and secondly, it offers a patient-centered approach to solidify the reasons that we are fitting the lenses and recommending the care systems that we are.
The Challenge of Store Brand Solutions
We are fortunate that contact lens wear is a relatively safe vision correction modality. For those who wear their lenses on a daily basis, the risk of developing a microbial keratitis is relatively low at about four in every 10,000 contact lens wearers.11,12 Sleeping in lenses increases the incidence about five times the risk of daily wear to about two in 1,000 contact lens wearers (figure 3).11,12 With such a high safety profile, do contact lens care systems matter?
Certainly, we cannot dismiss several other sequelae that can result from various solution use. Karen Yeung, O.D., and colleagues interviewed patients utilizing various contact lens care systems and found that patients who used store brand solutions had the highest rates of complications.13 The most common complications were giant papillary conjunctivitis, papillae and corneal neovascularization (figure 4). Although the number of patient using store brand solutions was low, the increased rate was significant.13 It is not clear whether these results show a true increase in complication rates in individuals using private label solutions or if it represents a population that is less likely to be compliant with contact lens care in general, predisposing them to complications through non-compliant care of their lenses.
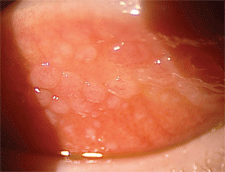 |
| 4. Giant papillary conjunctivitis. |
We have seen a number of patients who have switched to store brand solutions after having their appointment return to our offices complaining of mild to moderate discomfort. A small change, like the type of solution used, can cause a patient to go from a comfortable to uncomfortable wearing experience, and often times, going back to the original recommended solution can ease away the discomfort.
What’s New in the Market?
With the increased complexity of today’s newest technology lenses, it is critical that we meet the demands with solutions specifically created and tested with the new materials. Remember that private label solutions are often technologies created and tested in the era of hydrogel lenses. In the last year itself, three new lens care systems have been introduced to the market
and tested with silicone hydrogel lenses. Here is an overview:
- Biotrue: First introduced in mid-2010, Biotrue (Bausch + Lomb) is preserved with two disinfecting agents—PHMB and polyquaternium—which, when combined, offer a high level of disinfection efficacy.14 Unlike other solutions, Biotrue contains hyaluronic acid (HA). While HA has long been present in artificial tears to treat dry eye symptoms, it was introduced to solutions in an attempt to improve a patients wearing experience. Studies show that HA remains present on the surface of a contact lens for up to 20 hours after the lens has been removed from the soaking solution.15
- RevitaLens Ocutec: First introduced to the market towards the end of 2010, RevitaLens Ocutec (Abbott Medical Optics) contains a unique surfactant— tetronic 904. The solution is preserved with two disinfecting agents—alexidine and polyquaternium-1—a profile that makes RevitaLens Ocutec robust when examining activity against microorganisms via stand-alone testing criteria.16 In addition, it has a high level of anti-Acanthameoba activity against both trophozoite and cyst forms of the organism.17
- Opti-Free Puremoist: Introduced in the summer of 2011, Opti-Free Puremoist (Alcon) has a robust disinfection platform, containing both polyquad and aldox.18
The increasing complexity of the contact lenses that we are currently prescribing has translated into the development of new contact lens care to support the unique features and characteristics of these lenses. The store brand solutions, created in an era of hydrogel lenses, may not be the best option when working with the new generation of silicone hydrogel lenses. Instead, consider the newest generation of solutions, which have been created in an era of silicone hydrogel lenses. Embracing these new-generation solutions through effectively communicating the technology and identifying those that are straying from your instructions will certainly enhance your patient’s wearing experience.
Mile Brujic, O.D., is a partner of Premier Vision Group, a four-location optometric practice in northwest Ohio. Jason R. Miller, O.D., M.B.A., is a partner at EyeCare Professionals of Powell, Ohio, and serves as an adjunct faculty member for The Ohio State University College of Optometry.
1. Potter B, Stiegemeier M, Movic W, et al. A Clinical Evaluation of Solutions. Rev Cornea Contact Lens. 2005 Nov.
2. Young G, Keir N, Jones S. Clinical evaluation of longterm users of two different contact lens care preservative systems. Poster presented at the British Contact Lens Association meeting, May 2008; Birmingham, UK.
3. Lin MC, Tatyana TF. Differences in Protein-Removal Efficiency Among Multi-Purpose Solutions. Paper presented at the Association for Research in Vision and Ophthalmology meeting, April 28, 2008; Ft. Lauderdale, Fla.
4. Nichols JJ. Market and survey data show that the industry remained largely unaffected in 2010 by the state of the economy. CL Spectrum. 2011 Jan. Available at:
www.clspectrum.com/article.aspx?article=105083 (accessed September 2011).
5. Dillehay SM, Miller MB. Performance of Lotrafilcon B silicone hydrogel contact lenses in experienced low-Dk/t daily lens wearers. Eye Contact Lens. 2007 Nov;33 (6 Pt 1):272-7.
6. Bergenske P, Long B, Dillehay S, et al. Long-term clinical results: 3 years of up to 30-night continuous wear of lotrafilcon A silicone hydrogel and daily wear of low-Dk/t hydrogel lenses. Eye Contact Lens. 2007 Mar;33(2):74-80.
7. Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with the use of a contact lens solution. JAMA. 2006;296(8):953-63.
8. Saw S, Ool P, Tan D, et al. Risk factors for contact lens-related Fusarium keratitis: a case-control study in Singapore. Arch Ophthalmol. 2007 May;125(5): 611-7.
9. Joslin C, Tu E, Shoff M, et al. The association of contact lens solution use and acanthamoeba keratitis. Am J Ophthalmol. 2007 Jun;144(2):169-80.
10. Dumbleton K, Woods C, Jones L, et al. Patient and practitioner compliance with silicone hydrogel and daily disposable lens replacement in the United States. Eye Contact Lens. 2009 Jul;35(4):164-71.
11. Dart JK, Radford CF, Minassian D, et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008 Oct;115(10):1647-54.
12. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008 Oct;115(10):1655-62.
13. Forister JF, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009 Jul;35(4):176-80.
14. Bausch + Lomb. Biotrue. Available at: www.biotrue.com (accessed September 2011).
15. Merchea M, Scheuer CA, Burke SE, et al. Performance of a novel multi-purpose lens care solution formulated with a hyaluronic acid conditioning agent. Optom Vis Sci. 2010;87:E-abstract 105201.
16. Abbott Medical Optics. RevitaLens Ocutec multipurpose \disinfection solution. Available at: www.amo-inc.com/products/corneal/multi-purpose-solution/revitalens-ocutecmulti-
purpose-disinfecting-solution (accessed September 2011).
17. Kilvington S, Powell H, Lam A, et al. Antimicrobial properties of a new multi-purpose contact lens disinfectant solution. Poster presented at the American Academy Optometry meeting, November 17-19, 2010; San Francisco.
18. Alcon. Opti-Free products. Available at: www.opti-free.com (accessed September 2011).
19. Davis J, Kettleson HA, Shows A, Meadows DL. A lens care solution designed for wetting silicone hydrogel materials. Poster presented at the Association for Research in Vision and Ophthalmology, May 2-5, 2010; Fort Lauderdale, Fla.
20. Senchyna M, Stauffer P, Davis J, et al. Characterization of a multipurpose lens solution designed for silicone hydrogel materials. Poster presented at the Association for Research in Vision and Ophthalmology, May 2-5, 2010; Fort Lauderdale, Fla.


