 |
Corneal Complications of Diabetes: What ODs Need to Know
Given the significant impact this condition can have on a patient’s quality of life, optometrists must have a comprehensive understanding on the potential ocular involvement.
By Kamila Mikos, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: November 15, 2024
Expiration Date: November 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists who want to recognize and address diabetic corneal complications.
Educational Objectives: After completing this activity, participants should be better able to:
Recognize the prevalence and impact of diabetes on eye health.
Identify anterior segment complications related to diabetes.
Assess corneal health and function among patients with diabetes.
Develop treatment and management plans for diabetic patients.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Dr. Mikos has no financial disclosures has no financial disclosures. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #129491 and course ID 94480-SD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
Diabetes mellitus, with its ever-growing prevalence, is one of the most common systemic diseases eyecare providers encounter. The International Diabetic Federation estimates that close to 537 million adults worldwide are living with diabetes, including 51 million in North America alone.1 The consequences of this chronic, metabolic disorder are well studied, including microvascular changes, nephropathy, retinopathy and neuropathy. These complications can severely impact a patient’s quality of life by increasing their morbidity and mortality. Most notably for eyecare providers, diabetes is the leading cause of blindness in the United States.
While ocular complications of diabetes tend to focus on diabetic retinopathy, the anterior segment of the eye is frequently overlooked. We are learning that the cornea and ocular surface are also significantly affected by diabetes; however, these complications are often underdiagnosed. Estimates suggest that approximately 70% of patients suffer from corneal complications.2 Therefore, a comprehensive approach to fully assess the ocular health in diabetic patients is crucial to manage the sequelae of this condition.
Understanding Pathogenesis
Hyperglycemia, the formation of advanced glycation end products, and oxidative stress result in a variety of anterior segment complications, including defective corneal epithelial healing, subbasal corneal nerve abnormalities and dysfunction of the corneal endothelial pump.3
Research shows that diabetes leads to an upregulation of insulin-like growth factor-binding proteins. These proteins compete with insulin-like growth factor, which plays a critical role in corneal wound healing and cell proliferation. When insulin-like growth factor becomes less available, epithelial cell damage occurs due to dysregulation. This can manifest as chronic epithelial defects that may result in recurrent erosions or delayed wound healing.
Oxidative stress and the accumulation of reactive oxygen species play a role in corneal cell damage and inflammation. Chronic inflammation perpetuates a cycle of damage, affecting corneal epithelial cells and nerves, which are important in proper wound healing and regeneration.3,4
 |
|
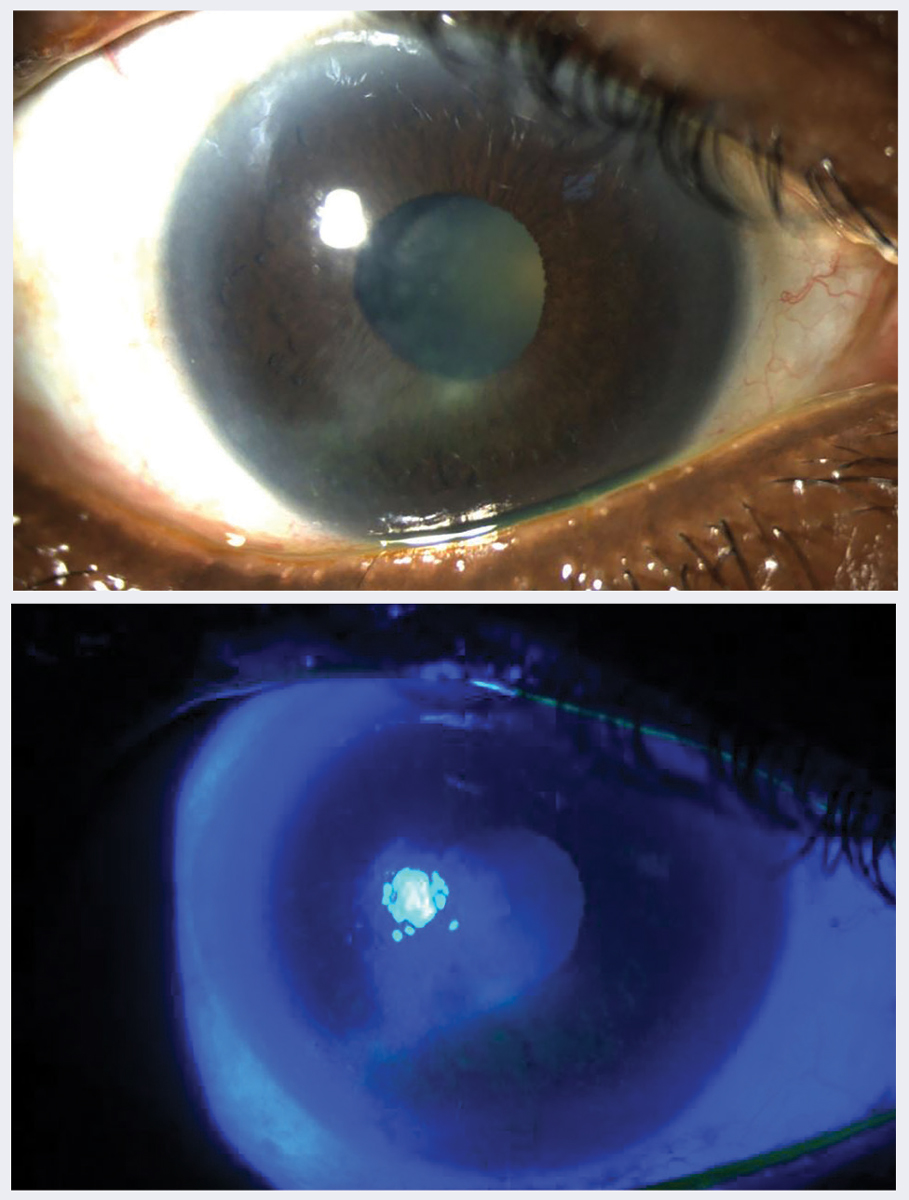
Fig. 1. (A) Neurotrophic keratitis in a 78-year-old woman with uncontrolled type 2 diabetes for 20 years, A1c 11%, proliferative diabetic retinopathy OU seen in white light and (B) under cobalt blue filter. Click image to enlarge. |
Understand the Causes
This condition is idiopathic in nature, so neurological workups should reveal no identifiable cause for the increased intracranial pressure. However, secondary intracranial hypertension has been associated with certain systemic medications and conditions. Systemic medications linked to secondary intracranial hypertension include tetracyclines, retinoids, excessive vitamin A and hormonal medications, such as tamoxifen.2 It is recommended to discontinue these medications if IIH develops following their initiation.7 Systemic conditions associated with IIH include renal failure, anemia, Addison’s disease, Cushing’s disease and sleep apnea. In cases of sleep apnea, which predominantly affects men, nocturnal hypoxia is believed to lead to cerebral vasodilation and increased blood flow, ultimately raising intracranial pressure.
Tear Film Dynamics
Essential for a healthy cornea, tears prevent infection, flush away debris and unwanted particles, keep the cornea moist and hydrated and create a protective cushion between the cornea and the environment. Hyperglycemia and the increase of advanced glycation end products can cause damage to the lacrimal gland, causing decreased tear production. Additionally, reduced corneal sensitivity, commonly seen in diabetic patients, can compromise tear production by diminishing the tear reflex, which consequently reduces reflex-induced lacrimal secretion and the production of basal tears.3
Not only can tear film quantity be affected, but the components of the tear film in diabetic patients may not also be working cohesively. Mucin, which is important in forming the glycocalyx that adheres the tear film to the cornea, is secreted by our corneal and conjunctival epithelial cells. Studies show that goblet cells, important for maintaining the mucin layer, can be reduced in patients with diabetes.5,6 When conjunctival cell damage occurs, this layer can be compromised, promoting friction and increasing the likelihood of damaging the fragile corneal epithelium.
The lipid layer of the tear film, which is important for preventing tear film evaporation, has been shown to be compromised in this patient population as well. The changes in the meibomian glands did not correlate to changes in symptoms score on the Ocular Surface Disease Index (OSDI) survey, possibly due to the changes in corneal sensitivity. The lipid layer may also be disrupted due to decreased corneal sensitivity, which reduces the blink rate and causes increased evaporation.7
Other factors in the tear film have been studied in relation to diabetes. Studies show increased matrix metalloproteinases and tear osmolarity in diabetic patients. Matrix metalloproteinases are an inflammatory marker found in the tear film, which may be increased due to hyperglycemia triggering inflammatory cascades that promote ocular surface disease. They can delay wound healing and promote anti-collagenase activity as well. Similarly, tear hyperosmolarity is seen more frequently in diabetic patients, propagating the inflammatory cascade as well.4
 |
|
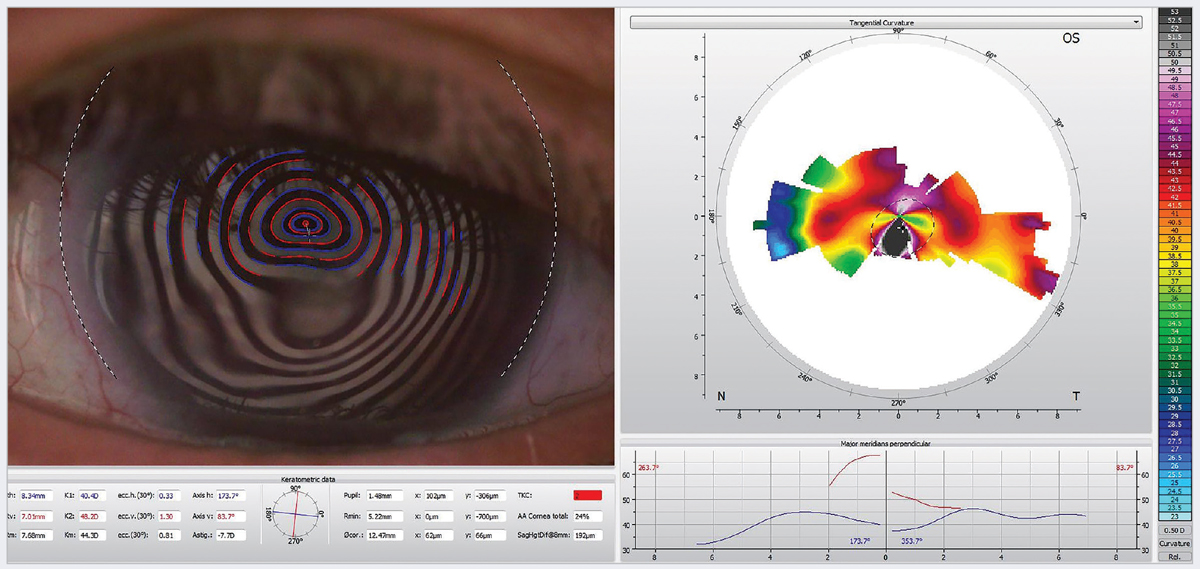
Fig. 2. Induced irregular astigmatism in a 70-year-old woman with neurotrophic keratitis. One treatment option for this patient is scleral contact lenses. Click image to enlarge. |
Corneal Epithelium
Substance P, an important neuropeptide that is a crucial component of neuronal support to the cornea, has been shown to be reduced in the tear film of diabetic patients. Lower levels of substance P can lead to decreased nerve fibers and impaired corneal wound healing. A fragile epithelium and weakened adhesions between the epithelium and the basement membrane complex put patients at a higher risk for epithelial defects, corneal erosions and infections.
One of the ways this translates for eyecare providers is an increased risk of contact lens-related complications in diabetic patients. They are more susceptible to contact lens-induced epithelial damage, reduced hydration and oxygen exchange and infectious keratitis. This can lead to issues ranging from increased contact lens discomfort to serious microbial keratitis; therefore, caution should be used when fitting diabetic patients.8
Other clinical considerations include refractive surgeries such as laser-assisted in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK). These procedures are a relative contraindication in patients with diabetes due to poorer refractive outcomes and epithelial complications, which occur in about 50% of cases.9
Since PRK involves removal of the epithelium, this can be problematic in patients that have impaired epithelial healing. LASIK may be preferable as it involves only creating a flap and applying laser directly to the stroma; however, it still causes epithelial damage, putting patients at higher risk of complications like epithelial ingrowth.2,10,11 Other factors to consider are damaged sensory nerves and goblet cells, which are already compromised in diabetic patients. These procedures may still be performed with caution while being particularly careful in patients with a high HbA1C or uncontrolled glucose control.
Corneal Stroma
The limited research on the corneal stroma in diabetic patients shows that collagen fibril crosslinking increases in response to hyperglycemia and the accumulation of advanced glycation end products. This is further exacerbated in patients with poor glycemic control and patients with proliferative diabetic retinopathy and can lead to an increase in central corneal thickness.12
Corneal Endothelium
Hyperglycemia leads to the accumulation of sorbitol and advanced glycation end products that may cause endothelial cell decompensation. This can result in decreased endothelial cell density, polymegathism (increased cell size and variation of size of cells) and pleomorphism (decrease in hexagonality of cells).13
One way to assess the corneal endothelium in clinic is through an endothelial cell count to pick up on these changes. Increased central corneal thickness may also be observed on pachymetry due to endothelial cell loss and increased collagen crosslinking in the stroma, leading to increased thickness and stiffness of the cornea. This has implications for patients, particularly after cataract surgery, where the endothelium’s role in maintaining corneal transparency and fluid regulation can be compromised.
Diabetic patients are more likely to suffer from postoperative complications such as corneal edema following cataract surgery.14 The endothelium—which is responsible for corneal deturgescence via regulating fluid transport—can be compromised in diabetic patients. Coupled with the mechanism trauma of the surgery, this can cause corneal edema, decreased visual acuity and a lack of transparency of the cornea.
 |
|
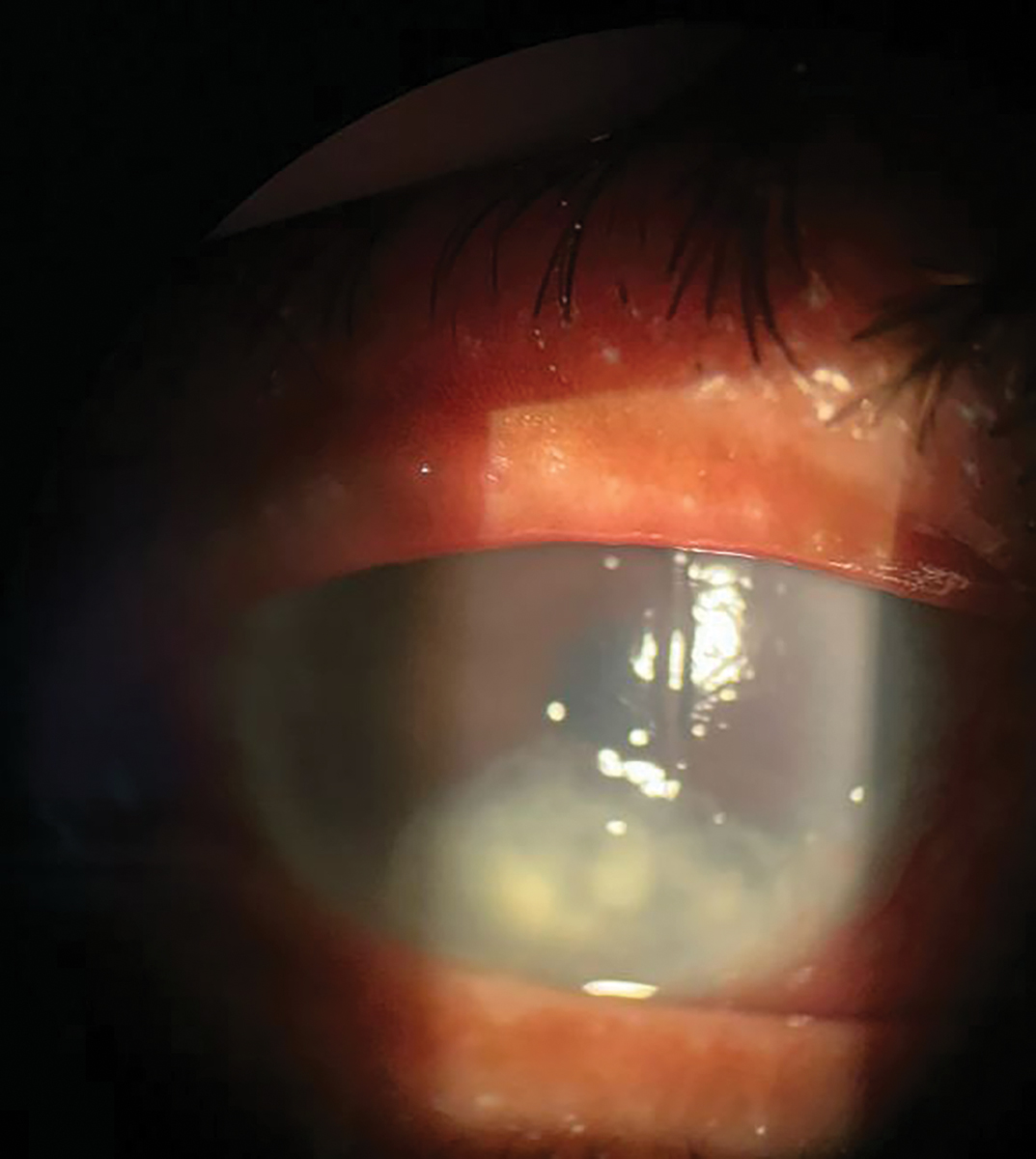
Fig. 3. Neurotrophic ulcer (stage 3 neurotrophic keratitis) in the right eye. There is an overlying epithelial defect with classic rolled edges in the inferior third of the cornea and underlying stromal haze. Click image to enlarge. |
Studies have shown that patients with diabetes account for 80% of cataract and refractive surgery corneal complications.4 Evaluation of the corneal endothelium is recommended prior to cataract surgery, especially in patients with uncontrolled diabetes.4
Corneal Nerves
Systemic neuropathy, associated with both type 1 and type 2 diabetes, is a hallmark of the disease, affecting about 50% of patients.5 The cornea, being one of the most innervated organs in the body with the highest density of nerve fibers, is understandably affected by diabetes.
Chronic hyperglycemia can cause damage to the trigeminal nerve, resulting in a loss of corneal innervation. The corneal nerves provide sensory feedback to help promote epithelial cell proliferation, blink reflex and tear production. This occurs via the neurotrophic role of the nerves because of neuromediators, such as nerve growth factor.
Nerve growth factor is crucial for epithelial regeneration and maintaining neuronal health through the promotion of neuronal repair mechanisms by secreting a variety of neuropeptides, growth factors and cytokines. In a hyperglycemic state, such as in diabetics, we see decreased neuromediators (i.e., nerve growth factor) that play an important neurotrophic role in promoting proliferation of corneal epithelial cells and maintaining corneal homeostasis.15 This causes decreased corneal innervation, epithelial cell breakdown and delayed wound healing. Therefore, the corneal nerves have two downstream effects—direct impact on the nerves themselves and on the corneal epithelium.16
Eyecare providers should recognize the neurotrophic component of dry eye disease. This can lead to neurotrophic keratitis, a condition that is divided into three stages. The first stage is characterized by punctate corneal epithelial defects that are superficial. Stage 2 is more severe, resulting in persistent epithelial defects, often in the classic oval-shaped epithelial defect with “rolled edges.”
Stage 3 neurotrophic keratitis is characterized by stromal involvement, leading to ulceration, stromal melting and eventually corneal perforation. This stage is more challenging to manage, which is why it is critical to identify this disease in its earlier phases. Interestingly, patients may not have any symptoms at all, except for blurred vision, due to decreased corneal sensation, sometimes referred to as “stain with no pain.”
Beyond neurotrophic keratitis, changes in the corneal nerves among diabetic patients can induce neuropathic pain.17 This condition is characterized as persistent ocular pain without significantly visible signs on clinical examination and is sometimes referred to as “pain without stain.”
Hyperglycemia, ocular surface disease and various factors are thought to increase sensitization of the peripheral neurons innervating the cornea.16,17 This results in increased central sensitization over time, where the central neurons become highly responsive to a similar magnitude of pain, even when the peripheral stimulus is taken away. The spontaneous activity translates as a pain sensation and causes heightened awareness of overall pain.
Considerations for Diagnosis
Optometrists can be more apt at catching anterior segment complications of diabetes by incorporating certain testing for these patients as well as those with dry eye. Corneal nerve testing should be considered as part of a dry eye workup, particularly when a patient’s symptoms do not align with the signs seen on clinical examination.
A simple, qualitative test to perform in-office involves testing corneal sensitivity to check for a reduced blink response. One can take a cotton wisp, dental floss or thread to check a patient’s blink reflex. Patients with a reduced or absent blink reflex likely have reduced corneal sensation.
While typically used in research, esthesiometers, such as the Cochet-Bonnet esthesiometer or the Belmonte esthesiometer, are an option for more quantitative tests. The Cochet-Bonnet uses a nylon monofilament with a constant diameter to quantify how much pressure is needed to elicit a blink response. The Belmonte esthesiometer is a noninvasive option, using air puffs of various pressures, temperatures and concentrations of carbon dioxide to assess corneal mechanical, chemical and thermal sensitivity, respectively. The Brill esthesiometer is a great handheld alternative for practitioners. It has shown consistent repeatability and is a non-contact option for testing corneal sensitivity in-office.18
In addition to corneal nerve testing, sodium fluorescein dye can help measure tear break-up time. Checking for epithelial defects is crucial in these patients. Schirmer’s tests can be useful for the assessment of reduced basal tearing when used with anesthetic. Without anesthetic, Schirmer’s tests can evaluate both reduced basal and reflex tearing.
Studies have shown that patients with diabetes have significantly lower tear break-up time and Schirmer test values compared with non-diabetic patients.6 Tear osmolarity and inflammatory markers tend to be elevated in diabetic patients; therefore, ODs should consider tests such as InflammaDry (Quidel) or TearLab (Trukera/Bausch + Lomb).
 |
|
Fig. 4. Superficial punctate keratitis and dry eye syndrome in the left eye seen in a 62-year-old man with type 2 diabetes. Click image to enlarge. |
In cases where neuropathic pain is suspected, particularly when a patient has minimal signs of dry eye on examination, the proparacaine challenge test is a quick diagnostic tool. If a patient reports little to no improvement in symptoms after the instillation of proparacaine, the patient may have some central sensitization of their pain or hyperalgesia, as the peripheral stimulus has been removed.
Treatment and Management
Effective management of corneal sequelae of diabetes begins by emphasizing the importance of tight glycemic control. Studies imaging the corneal nerve plexus have shown an increase in the density of nerves with improvement of HbA1C, suggesting that lower HbA1C levels are associated with corneal nerve regeneration.19
Due to prolonged wound healing in diabetic patients, more aggressive dry eye treatments are necessary to promote corneal re-epithelialization. Providers should be aggressive with lubricants, including artificial tears and ointments; however, they should avoid preservatives that can exacerbate the condition. Punctal plugs should be used with caution with ocular surface inflammation, as the retention of inflammatory markers in the tear film may worsen dryness.20 Optometrists should also consider eye masks and eye shields to promote healing at night.
Therapeutic bandage contact lenses may help patients with persistent epithelial defects and recurrent corneal erosions. These lenses act as a barrier for the cornea, block friction from the eyelid and allow epithelial cells to adhere to each other. Another option is scleral lenses, which can create a barrier and lubricate the cornea constantly by allowing it to bathe in a reservoir of fluid. They also help correct any irregular astigmatism secondary to corneal complications. The FDA recently approved the PROSE lens (Prosthetic Replacement of the Ocular Surface) for therapeutic use for patients with corneal dystrophies and degenerations.21 This device, similar to a scleral lens, helps treat underlying ocular surface disease while improving vision.
Prophylactic topical antibiotic treatments should be considered for patients with persistent epithelial defects. It is strongly recommended to use this treatment with therapeutic contact lenses to decrease infection risk. Systemic antibiotics, particularly tetracyclines, can be considered for prophylaxis since they have the added benefit of helping inhibit matrix metalloproteinases that promote inflammation and help prevent collagen degradation.22
Au-tologous serum contains a variety of factors, such as vitamin A, growth factors and anti-inflammatory factors, that help with epithelial healing and nerve regeneration. Amniotic membranes, like cryopreserved or dehydrated, provide similar factors important for cell and nerve regeneration while also acting as a scaffold to promote corneal healing.
In the last few years, Oxervate (cenegermin-bkbj 20mcg/mL, Dompé), a topical medication, has been FDA-approved for treating the root cause of neurotrophic keratitis. Cenegermin is a recombinant human nerve growth factor, structurally similar to the human nerve growth factor responsible for promoting nerve and corneal regeneration. This novel medication has been shown in trials to stimulate corneal epithelial cell proliferation and differentiation as well as help with tear secretion.
The REPARO and NGF0214 trials demonstrated complete corneal healing in 72% of patients after an eight-week course of Oxervate that was sustained in 80% of patients 48 weeks (about 11 months) post treatment.23,24 Additionally, the study demonstrated improved corneal sensitivity and tear film production. This therapy aims to address the underlying cause of neurotrophic keratopathy, including cases secondary to diabetes.
For patients with neuropathic ocular pain, there are a variety of potential treatment options. Autologous serum drops and amniotic membranes have components, such as nerve growth factor, that aid in nerve regeneration to help alleviate symptoms. Since inflammation is believed to contribute to this condition, anti-inflammatory treatment, including a pulse dose with topical steroids or cyclosporine drops for more long-term use, can be beneficial.
Systemic pharmacotherapy has been used for central sensitization. Studies show that therapies, such as tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs) and anti-epileptic medications (i.e., pregabalin and gabapentin), have some efficacy in alleviating symptoms in patients with neuropathic pain.15
Severe cases may require surgical intervention. For instance, recurrent corneal erosions may necessitate phototherapeutic keratectomy, amniotic membrane transplantation, surgical tarsorrhaphy or even corneal transplantation to restore corneal integrity. Early detection of corneal complications can help prevent the need for these more aggressive treatments.
For neuropathic ocular pain, botulinum A toxin injections along the trigeminal nerve have relieved symptoms in some patients. In a study using botulinum A toxin for migraines, patients reported reduced ocular pain and photophobia in addition to relief of migraine symptoms. Injecting botulinum A toxin into the levator palpebrae superioris can serve as a temporary tarsorrhaphy for patients with severe signs, although it has visual and cosmetic implications.
Permanent tarsorrhaphy is rarely performed now due to the advancement of new treatment options for corneal disease. Corneal neurotization is reserved for severe cases of neurotrophic keratitis.25 This involves surgical nerve grafting of healthy nerves to reinnervate the anesthetic cornea and has had significant success. Additionally, many new treatments, including topical naltrexone therapies and thymosine beta 4, are currently under investigation and hold promise for these advanced corneal conditions.
Takeaways
As we gain more insight into diabetic keratopathy, novel treatments addressing the underlying disease process can help many patients with this condition. Ongoing research is necessary to enhance our treatment of the various corneal complications associated with diabetes mellitus. In practice, the early recognition of signs and symptoms is crucial for the preservation of patients’ vision and improving their quality of life.
Diabetic corneal complications initially present with subtle signs that can be easily overlooked, especially since diabetic eye examinations mainly focus on the posterior segment. Timely intervention can prevent severe corneal damage, such as ulceration and perforation, which require more severe therapy. Optometrists play a key role in managing patients with diabetes and ensuring their overall well-being and quality of life; thus, we must be equipped to recognize and address these complications when they arise.
1. International Diabetes Federation. IDF Diabetes Atlas. diabetesatlas.org/. Accessed October 15, 2024. 2. Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. Biomed Res Int. 2016;2016:3801570. 3. Shih K, Lam KL, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7(3):e251. 4. Ljubimov AV. Diabetic complications in the cornea. Vision Res. 2017;139:138-52. 5. Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18(2):168-74. 6. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108(3):586-92. 7. Lin X, Xu B, Zheng Y, et al. Meibomian gland dysfunction in type 2 diabetic patients. J Ophthalmol. 2017;2017:3047867. 8. O’Donnell C, Efron N. Diabetes and contact lens wear. Clin Exp Optom. 2012;95(3):328-37. 9. Fraunfelder FW, Rich LF. Laser-assisted in situ keratomileusis complications in diabetes mellitus. Cornea. 2002;21(3):246-8. 10. Jabbur NS, Chicani CF, Kuo IC, et al. Risk factors in interface epithelialization after laser in situ keratomileusis. J Refract Surg. 2004;20(4):343-8. 11. Han SB, Yang HK, Hyon JY. Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging. 2019;14:53-63. 12. Mocan MC, Durukan I, Irkec M, et al. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25(7):769-73. 13. El-Agamy A, Alsubaie S. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin Ophthalmol. 2017;11:481-6. 14. Hugod M, Storr-Paulsen A, Norregaard JC, et al. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 2011;30(7):749-53. 15. Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology. 2017;124(11S):S34-47. 16. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956-62. 17. Schreiber AK, Nones CF, Reis RC, et al. Diabetic neuropathic pain: Physiopathology and treatment. World J Diabetes. 2015;6(3):432-44. 18. Merayo-Lloves J, Gómez Martín C, Lozano-Sanroma J, et al. Assessment and safety of the new esthesiometer BRILL: comparison with the Cochet-Bonnet Esthesiometer. Eur J Ophthalmol. 2024;34(4):1036-45. 19. Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2012;62(1):254-60. 20. Jehangir N, Bever G, Mahmood SMJ, Moshirfar M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. J Ophthalmol. 2016;2016:9312340. 21. Mian SZ, Agranat JS, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem (PROSE) treatment for complications after LASIK. Eye Contact Lens. 2016;42(6):371-3. 22. Ogut D, Reel B, Korkmaz CG, et al. Doxycycline down-regulates matrix metalloproteinase expression and inhibits NF-KB signaling in LPS-induced PC3 cells. Folia Histochem Cytobiol. 2017;54(4):171-80. 23. Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332-43. 24. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14-26. 25. Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123(1):112-20. |


