 To build a better lubricant is the holy grail of ocular surface science. Ideally, a lubricant should address all the components of the natural tears—mucin, water and lipid layers. The lipid layer protects against evaporation. The aqueous portion forms a gel with soluble mucins, and the mucin layer helps spread the aqueous gel over the ocular surface. Finding the right combination of agents in an artificial tear, in a long-lasting formulation, is not an easy task.
To build a better lubricant is the holy grail of ocular surface science. Ideally, a lubricant should address all the components of the natural tears—mucin, water and lipid layers. The lipid layer protects against evaporation. The aqueous portion forms a gel with soluble mucins, and the mucin layer helps spread the aqueous gel over the ocular surface. Finding the right combination of agents in an artificial tear, in a long-lasting formulation, is not an easy task.
How It Works
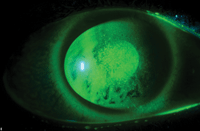
A rapid tear break up time due to insufficient lipid layer.
Some liquids molecules won’t mingle because of the difference in attractions. For example, the attractions between water molecules are very strong, whereas oil particles are only weakly held together. The oil molecules can’t overcome the strong attractions between the water molecules, so the oil will sit on top of the water. Liquids that do not mix with each other, such as oil and water, are immiscible.
Although some pairs of liquids are immiscible, we can force them together in an emulsion. Instead of forming two separate layers with a clear boundary between them, small droplets of one liquid are spread throughout the other liquid. This can be either a water-in-oil or an oil-in-water mixture.
The theory here is that a few, bigger droplets will have less surface area than many little ones. The less surface area, the less the two liquids have to touch each other—their preferred state. When an emulsion is shaken very hard, the droplet size is made smaller (i.e., salad dressing). Shaking, however, is an inefficient way of making an emulsion. Instead, to keep an emulsion from separating, we add a surfactant—a surface active agent, otherwise known as an emulsifying agent.
A surfactant includes a hydrophilic and a hydrophobic portion. The hydrophilic end is water-soluble, while the hydrophobic end is water insoluble or lipophilic. Surfactants align accordingly between the water and oil interfaces, and reduce surface tension. These emulsifying agents absorb quickly around dispersed drops as a condensed, non-adherent film, which prevents coalescence. They impart adequate electric potential so that mutual repulsion occurs.
The small droplets in an emulsion scatter the light passing through it. The result is that the emulsion appears to be either an opaque grey or white. This effect is similar to a bowl of sugar: each individual grain is transparent, but a collection of grains appears white. Emulsion lubricating drops quickly separate upon instillation in the eye, producing only a momentary visual blur. The oil and water components merge with the natural tear layers.
In addition to the air and tear interface, the cornea and tear interface is also important to tear coverage. A hydrophobic ocular surface will cause tear film instability and rapid tear break-up times. A demulcent is an agent that forms a film over a mucous membrane, and is sometimes referred to as mucoprotective or mucomimetic agent. Mucomimetic agents include polyethylene glycol, propylene glycol, hydroxymethyl cellulose, sodium hyaluronate and guar.
Tear-film break-up time (TBUT) is a common clinical test of dry eye conditions. A quick TBUT has been implicated as a mechanism of ocular surface damage. The TBUT positively correlates with the lipid layer thickness and ocular surface hydrophilicity.1
There are currently only two emulsion products available on the market: Systane Balance (Alcon) and FreshKote (Focus). We, as practitioners, should be clinically prudent and focus dry eye treatment on increasing tear film stability.
1. Isreb MA, Greiner JV, Korb DR, et al. Correlation of lipid layer thickness measurements with fluorescein tear break up time and Schirmer’s test. Eye. 2003 Jan;17(1):79-83.


