Visual rehabilitation with contact lenses for patients who suffer from irregular corneas was a hit or miss, and often frustrating, endeavor. These patients typically were told that the only option available to them was corneal gas permeable contact lenses (GPs).
For fittings, practitioners used keratometry findings as a starting point and followed up with diagnostic GPs and a fluorescein evaluation. With the advent of today’s advanced technologies, we are able to measure the shape of the cornea and the ocular surface with high precision, thus providing a far greater understanding of the disease state and an improved direction to better fit our patients.
We can also now evaluate visual performance with and without contact lenses in ways that were only available in the optics laboratory in the past. This article highlights cases that have incorporated many of the available advanced ophthalmic technologies that have made great strides in clinical success and efficiency.
Placido-based Corneal Topography
This imaging technology uses information processed from concentric rings reflected off of the anterior ocular surface in order to calculate corneal curvature. Since the reflection is actually off of the tear layer, its stability or instability will have a dramatic effect on the outcomes. The separation of the concentric rings helps calculate inferred corneal curvature. The closer the rings are to each other, the steeper the curvature is at that location. The cumulative analysis of corneal curvature data then creates a topography map. Curvature data is an excellent way to infer the optical characteristics of the visual system since the majority of refraction takes place at the anterior ocular surface interface.
Evident in these subsequent case examples, one can analyze curvature data of the anterior cornea in order to predict refractive performance. Still, there are some significant limitations to information obtained from Placido-based corneal topography that often hinder our understanding of the disease state. These include, among others, an inability to measure the posterior cornea or the global corneal thickness.
Anterior segment OCT and Corneo-scleral Profiling
Tomography is a two-dimensional representation of a three-dimensional structure. Ocular tomography provides imaging and analysis of multiple “slices” of the cornea and anterior segment. Specifically, Scheimpflug tomography uses a photographic imaging technology to provide a 360° analysis.
Anterior segment ocular coherence tomography (AS-OCT) can also provide similar imaging with even greater resolution in order to measure such structures as the epithelial corneal thickness. The two technologies are able to measure anterior and posterior corneal surfaces as well as a global area of corneal thickness.
Corneo-scleral profile analysis provides a detailed description of the anterior surfaces of the cornea and the sclera (Figure 1). With the increased popularity of scleral lenses, corneo-scleral profiling is revolutionizing our understanding of anterior segment shape and our ability to design lenses that contour, with great precision, a surface that is now known to be quite asymmetric.
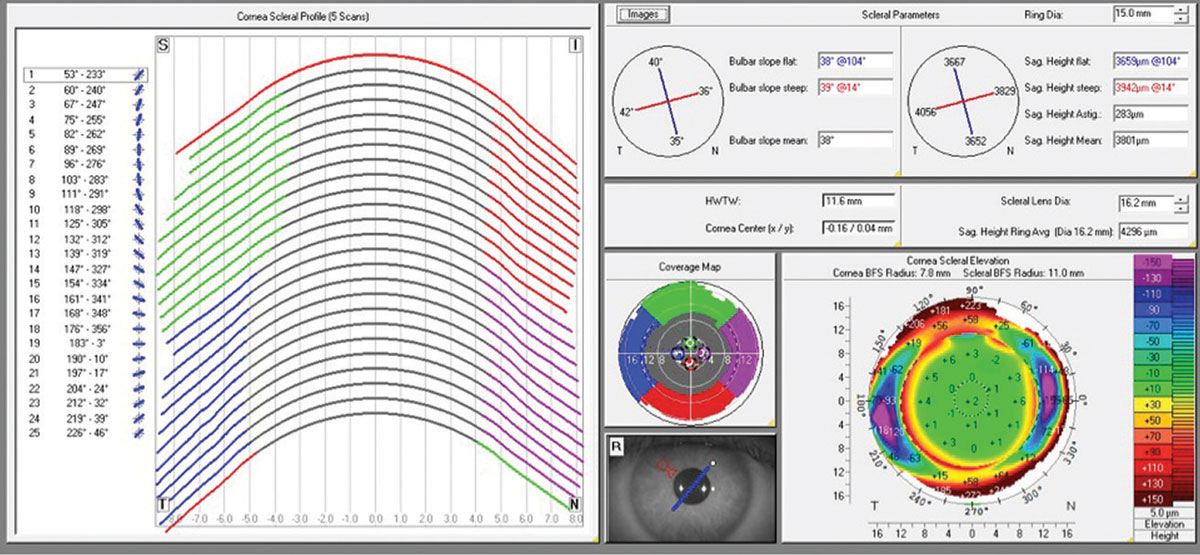 | |
| Fig 1. Corneo-scleral profile software from Pentacam tomography system. Click image to enlarge. |
Case 1: Is it PMD?
A 53-year-old woman was referred to our practice from a local optometrist for advanced contact lens management based upon a suspected diagnosis of pellucid marginal degeneration (PMD). The optometrist found significant against-the-rule astigmatism and performed Placido-based corneal topography, which reportedly found what has been described as a “crab claw” or a “kissing dove” pattern. Physical examination of the patient revealed no evidence of inferior thinning in the perilimbal zone of the cornea. There was evidence of mild Vogt’s striae (grade 1) located inferiorly and paracentrally along with a mild and partial inferior Fleischer’s ring. No corneal scarring was found. Manifest refraction revealed a highly myopic astigmatic refractive error (-5.00 -6.00x70) with a best-corrected visual acuity of 20/20-2.
In order to better understand the corneal condition, Scheimpflug-based corneal tomography was performed using the Pentacam (Oculus) instrument (Figure 2). Looking at the axial curvature display first revealed PMD’s classic crab claw/kissing dove pattern; however, the elevation displays on the anterior and posterior cornea were quite typical of keratoconus. More importantly, the global pachymetry display showed that the thin point of the cornea was located coincident with the apex of the cone in an inferior paracentral position. In true PMD, the thinning of the cornea would be located inferiorly in the far periphery of the cornea, corresponding to an area about one to two inches from the limbus.
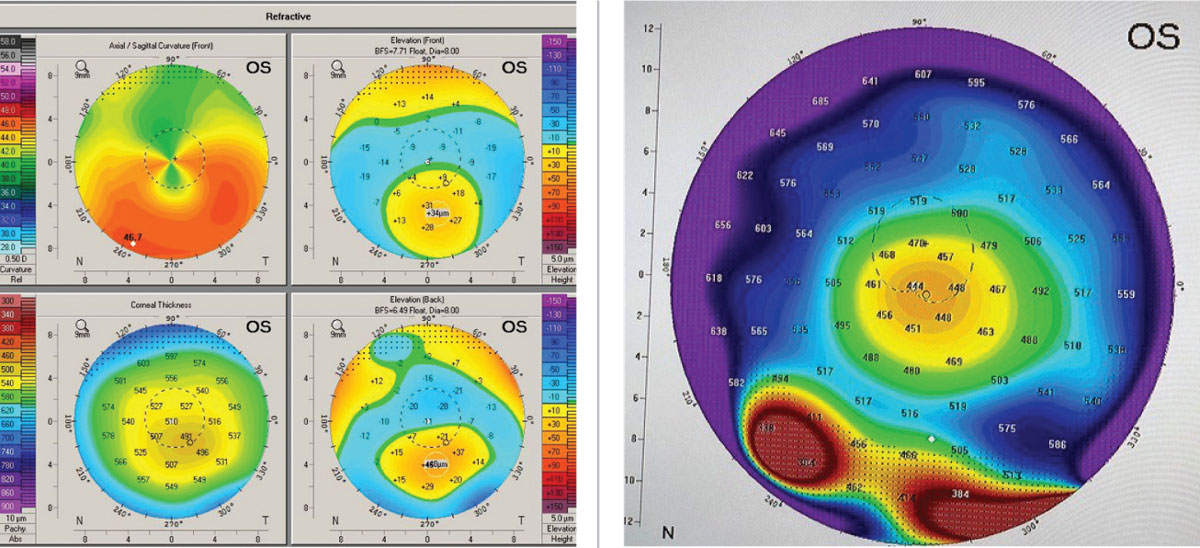 |
| Figs 2 and 3. (Left) The upper right axial or curvature map of the anterior cornea shows the “crab claw” or “kissing dove” pattern. (Right) Global pachymetry display from Pentacam with area in true PMD shows the band of inferior peripheral corneal thinning. Images: Tom Arnold, OD. Click image to enlarge. |
Another interesting observation in this case is the regularity of corneal curvature within the pupillary zone. Although six diopters of corneal astigmatism was measured by Pentacam via “Sim Ks,” there was almost no irregularity to the pattern. As such, it was no great surprise that we were able to achieve very good visual acuity with manifest refraction. Contact lens management then allowed my team to consider using soft contact lenses. In fact, we were able to fit the patient in a custom toric multifocal contact lens (SpecialEyes near center progressive toric multifocal) and obtain 20/20 distance and 20/25 near visual acuity.
Scheimpflug corneal tomography provides true elevation data from the anterior and posterior corneal surfaces as well as corneal curvature data (derived from elevation measurements). Additionally, it is able to measure global corneal thickness from limbus to limbus. This technology allows the clinician access to comprehensive information about the entire corneal structure. Beyond corneal measurements, Scheimpflug corneal tomography is also able to image out to the scleral surface (providing corneo-scleral profile data) and posteriorly as well to provide information and data regarding the anterior chamber, iris and crystalline lens.
This case is an excellent example of the vast majority of instances where anterior corneal curvature maps show what was formerly thought to be PMD. From clinical experience, the overwhelming majority of cases with these curvature patterns have actually been true keratoconus when we look at elevation and global pachymetry results found from corneal tomography. One study described this phenomenon exquisitely.1 When true PMD is found, the clinician will see a band of inferior corneal thinning typically located 1mm to 2mm in from the inferior limbus.1 In addition, if one expands the area displayed on the global pachymetry map from a typical 8mm or 9mm to 12mm in cases of true PMD, one will now see the band of inferior thinning found in this disease (Figure 3). Wavefront aberrometry has also been suggested as a means to differentiate keratoconus from PMD. One study assessed higher-order aberrations and found greater amounts of vertical coma in keratoconus and greater amounts of trefoil in patients with PMD.2 Another suggested that increases in coma-like aberrations of the cornea reflect the subclinical progression of PMD over the years.3
Cases 2 & 3: Is it Unilateral Keratoconus?
A 57-year-old female was referred for contact lens management from an ophthalmology group based on a /diagnosis of unilateral keratoconus of the right eye. The referring doctor performed no corneal imaging. Diagnosis was based on biomicroscopy and manual keratometry in addition to a positive family history of keratoconus. Biomicroscopy revealed Fleischer’s ring OD and grade 1 Vogt’s striae (no scaring) OD and entirely normal OS.
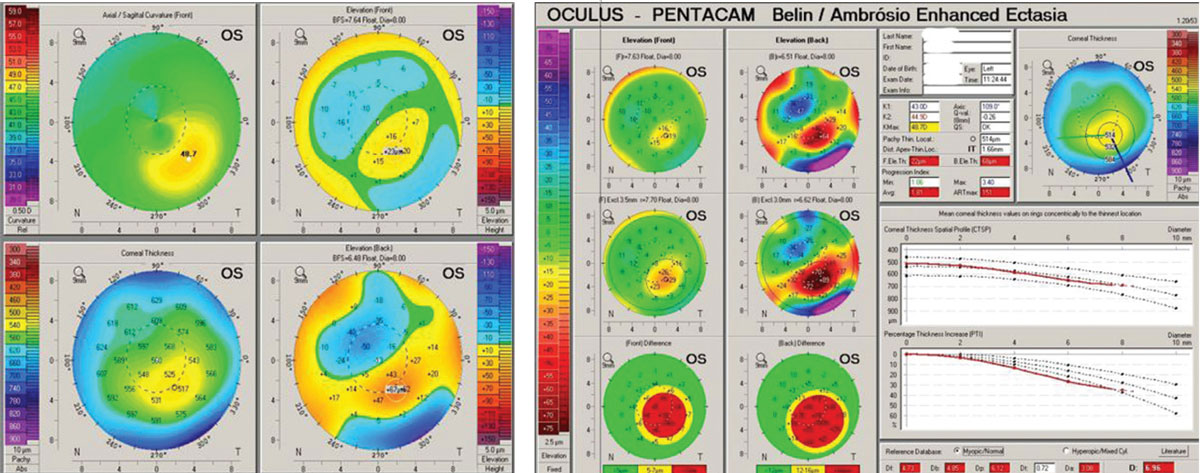
Manifest refraction found best spectacle acuity OD at 20/30+ and OS at 20/15. Scheimpflug corneal tomography (Pentacam) revealed OD a classic keratoconus pattern of mild-moderate degree. The OS image revealed an inferior steep zone on anterior curvature but a normal central area. Elevation maps found a normal anterior elevation but an abnormal posterior elevation along with a borderline abnormal progression of global corneal thickness from center to periphery (Figure 4).
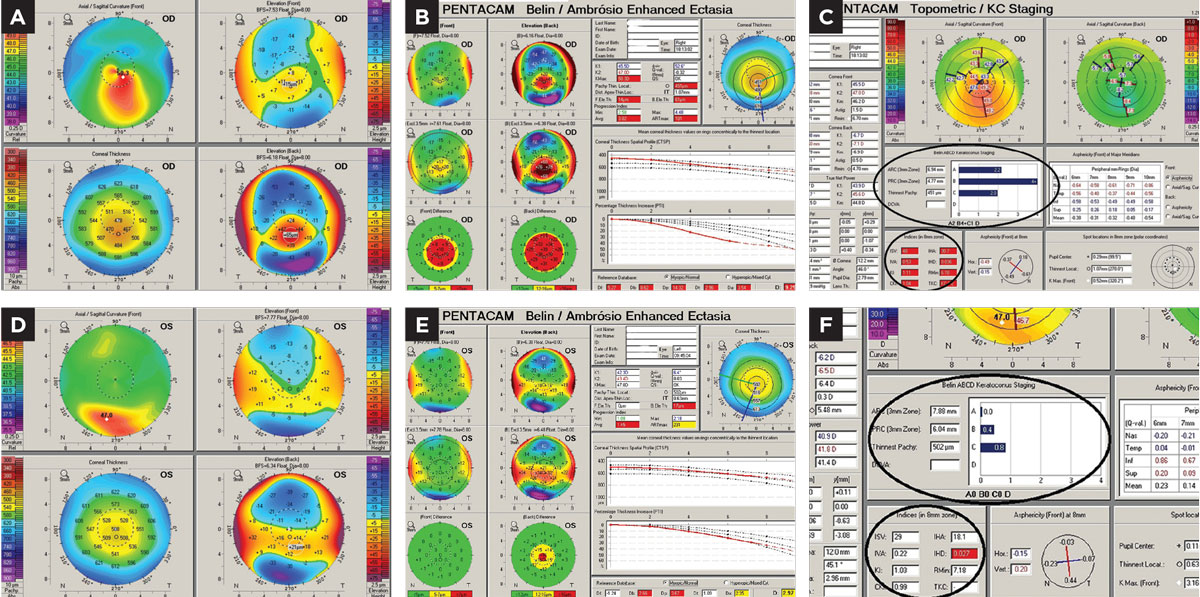 |
| Fig 4. (a) Pentacam 4-Map Refractive Display OD with keratoconus patterns. (b) Pentacam Belin Ambrosio Ectasia Display OD with strongly positive ectasia detection. (c) Topometric Display OD shows strong keratoconus staging and abnormal keratoconus indices derived from the anterior corneal surface. (d) Refractive Display OS with inferior axial steepening, normal anterior elevation, mildly abnormal posterior elevation and what would appear to be normal corneal thickness. (e) Ectasia Display OS shows normal anterior elevation, abnormal posterior elevation and borderline corneal thickness distribution/progression with a statistically normal minimal corneal thickness reading. (f) Topometric display OS shows normal keratoconus staging values and anterior corneal keratoconus indices. Click image to enlarge. |
Abnormal posterior corneal shape and abnormal corneal thickness distribution or thickness progression are considered two of three critical diagnostic findings for keratoconus.4 Aberrometry (Nidek OPD-Scan III) measured visual performance and found significant elevation of high-order aberrations (anterior corneal, internal and total aberrations) OD; however, OS had normal levels of high order aberrations, thus corroborating the excellent vision quality found with manifest refraction (Figure 5).
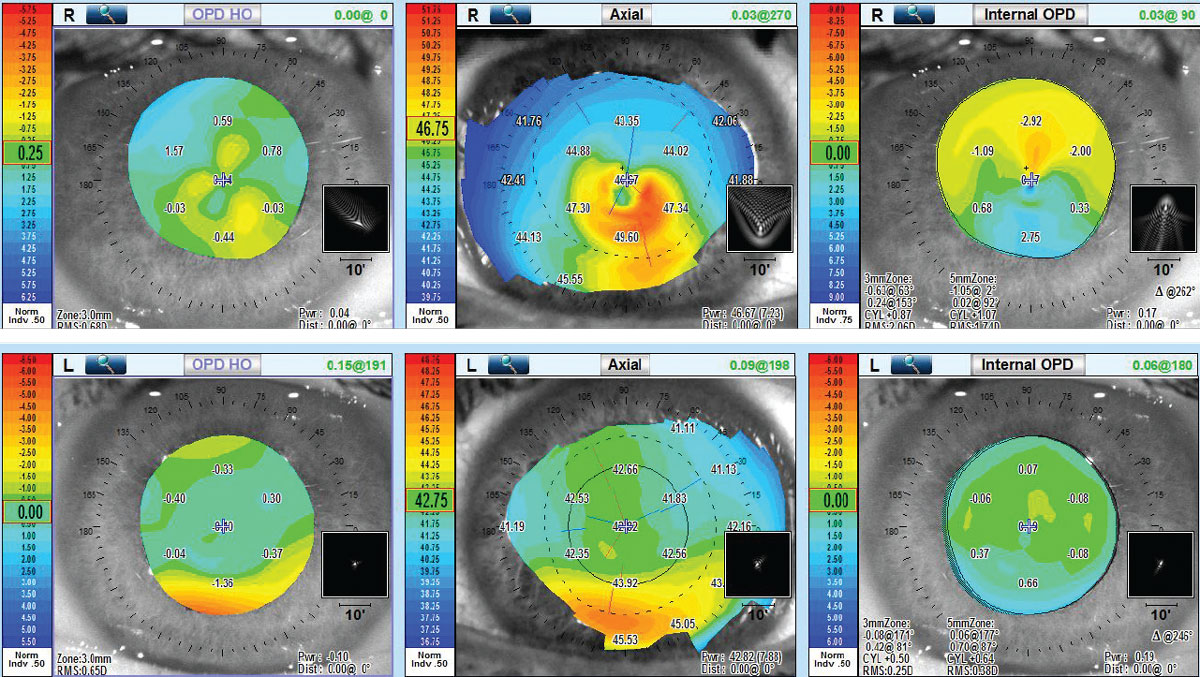 |
| Fig 5. (Top) Aberrometry OD shows elevation of high-order aberrations of total, anterior corneal and internal. (Bottom) Aberrometry OS shows normal high-order aberration findings of all three. Click image to enlarge. |
Aberrometry detects high-order aberrations that are found in keratoconus and can be present even in the presence of 20/20 visual acuity. As such, aberrometry has been shown to be influenced by early keratoconus as long as there is some shape anomaly of either the anterior or posterior cornea within the pupillary zone. In this case, corneal shape OS was entirely normal within the pupillary zone. The patient was subsequently successfully fit into corneal GP lenses with a posterior aspheric shape to provide presbyopic correction. Though it may be considered normal, the left eye surely suggests that the condition is bilaterally asymmetric. Based on the patient’s age, there was a relatively low risk for progression, but annual monitoring of tomography has been performed.
A 42-year-old male was examined who had also been diagnosed and managed with what was thought to be unilateral keratoconus of the left eye for a number of years. Based on sequential Scheimpflug corneal tomography (Pentacam), there was no demonstrable progression in the left eye and no development of clinically detectable keratoconus in the left eye over this period of time (Figure 6). However, performance of AS-OCT allowed us to measure corneal epithelial thickness and produce an epithelial thickness map.
 |
| Fig 6. (Left) Pentacam OS 4-Map Refractive Display shows classic keratoconic patterns. (Right) Ectasia Display OS shows strongly positive ectasia detection. Click image to enlarge. |
Variability of epithelial thickness across the cornea has been shown to be significantly greater in keratoconus compared with normal corneas.5 A typical pattern often develops where there is epithelial thinning over the cone apex with a surrounding “donut” of epithelial thickening, but epi-thickness variability will show, earlier in the disease, an increase even before developing this pattern. In this case, although the Pentacam was found to be significantly abnormal OS and normal OD, there was a noted asymmetric bilateral increase in epi-thickness variability found with AS-OCT (Figure 7). Continued work in this area will tell us whether this indication of bilateral keratoconus might be found earlier in the course of the disease.
With the advent of corneal crosslinking and our ability to control keratoconus progression, early detection of disease and of progression have become critical in our ability to preserve vision. Detection of the disease and of its progression are dependent on both our definition of the disease and available diagnostic technologies.
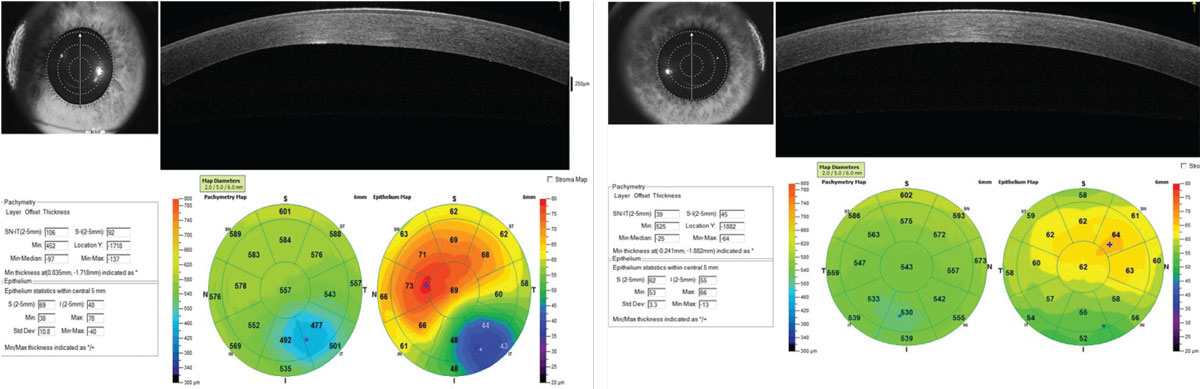 |
| Fig 7. Pachymetry Display. (Left) OS shows abnormal global corneal thickness and a classic pattern of thinning of the epithelium over the cone apex and surrounding epithelial thickening. (Right) OD shows normal global corneal thickness but abnormal epithelial thickness distribution. Click image to enlarge. |
Decades ago, classic biomicroscopic findings, scissors reflex with retinoscopy and distorted manual keratometry detected keratoconus. In fact, reports on the prevalence of keratoconus based on these diagnostic criteria likely have dramatically underestimated how common the condition actually is.6 With the introduction of Placido-based corneal topography, eye care practitioners began to detect keratoconus at earlier stages where former diagnostic criteria were absent. Subsequent studies of keratoconus prevalence that included topographic findings resulted in significantly higher rates.7
Today we are developing diagnostic technologies, such as Scheimpflug corneal tomography, AS-OCT with epithelial thickness mapping and clinically applicable aberrometry, that further push the boundaries of early detection. Beyond this, efforts are being made in corneal biomechanics and genetic screening to identify patients with pre-clinical keratoconus and those at high risk for its development.8,9
Case 4: Out to the Conjunctiva
A 64-year-old Caucasian male was referred by a cornea group and their affiliated optometrist to manage a case of scleral lens intolerance of the right eye. The patient had a history of bilateral radial keratotomy (RK). He initially had good vision for over 20 years but gradually noticed that his right eye vision had become progressively blurry and distorted with increased glare and light sensitivity. The patient had a secondary LASIK procedure and developed what he said was a post-operative “infection” that resulted in progressive visual distortion. Subsequently, he developed bilateral cataract and had surgery.
Scheimpflug tomography showed non-orthogonal irregular astigmatism and significant anterior corneal elevation asymmetry but no evidence of ectasia. Mapping of the global pachymetry and epithelial thickness found significant epithelial thickness variation but no evidence of post-surgical ectasia. Specular microscopy revealed a reduced cell count and cellular morphological anomalies of size and shape; however, the cell count was over 1,000. As such, concerns for fitting a scleral lens in terms of oxygen transmissibility existed but did not absolutely contraindicate scleral lens wear (Figure 8).
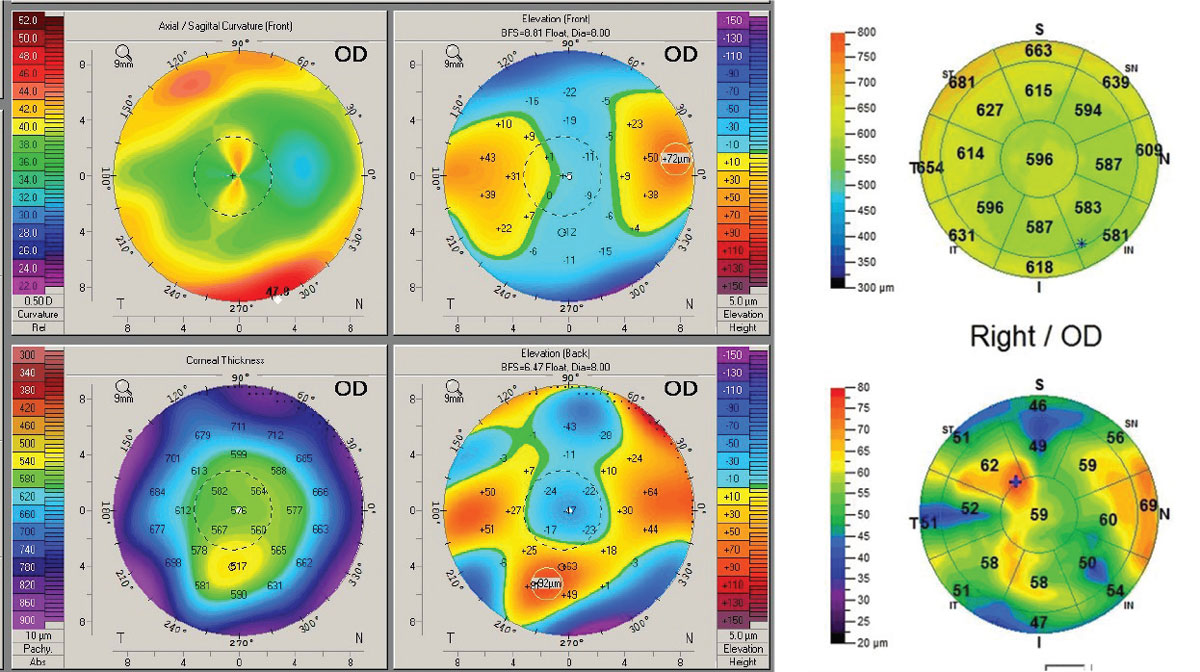 |
| Fig. 8. Post-RK/LASIK. (Left) Pentacam 4-Map Refractive Display shows non-orthogonal irregular astigmatism within the pupillary zone and significant anterior elevation asymmetry. (Right) OCT pachymetry map shows significant irregularity of the epithelial thickness but no evidence of ectasia. Click image to enlarge. |
The patient was fit in multiple traditional scleral designs but had noticed progressive discomfort and reduced wearing time. Ultimately, the patient was referred to our practice for consideration of impression scleral prosthetic treatment (EyePrintPro). The impression was scanned using a 3D printing scanner that created a detailed corneo-scleral 3D model, from which an initial prosthetic device was designed (Figure 9).
At follow-up after initial dispensing, biomicroscopy discovered areas of inferior/inferior-temporal conjunctival injection. The patient reported progressive lens awareness in the associated areas as wearing time increased. Careful observation noted inferior and temporal conjunctivochalasis and inferior/ inferior temporal entrapment of redundant conjunctiva under the lens edge (Figure 10). Numerous attempts at design modification failed to resolve the problem. A referral was made to our oculoplastic surgeon, who performed conjunctival patch removal with amniotic graft to address the chalasis. Following healing, reapplying the EyePrint device provided significantly improved comfort and vision.
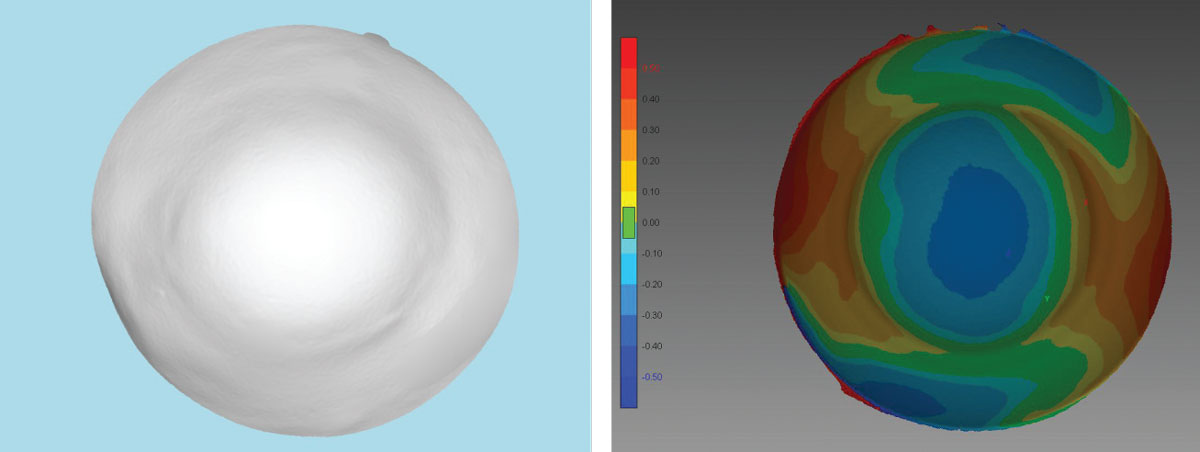 |
| Fig 9. (Left) A 3D printed model from an initial EyePrint impression is used to design the customized prosthetic. (Right) This corneo-scleral profile map is also constructed from the 3D printed impression. Click image to enlarge. |
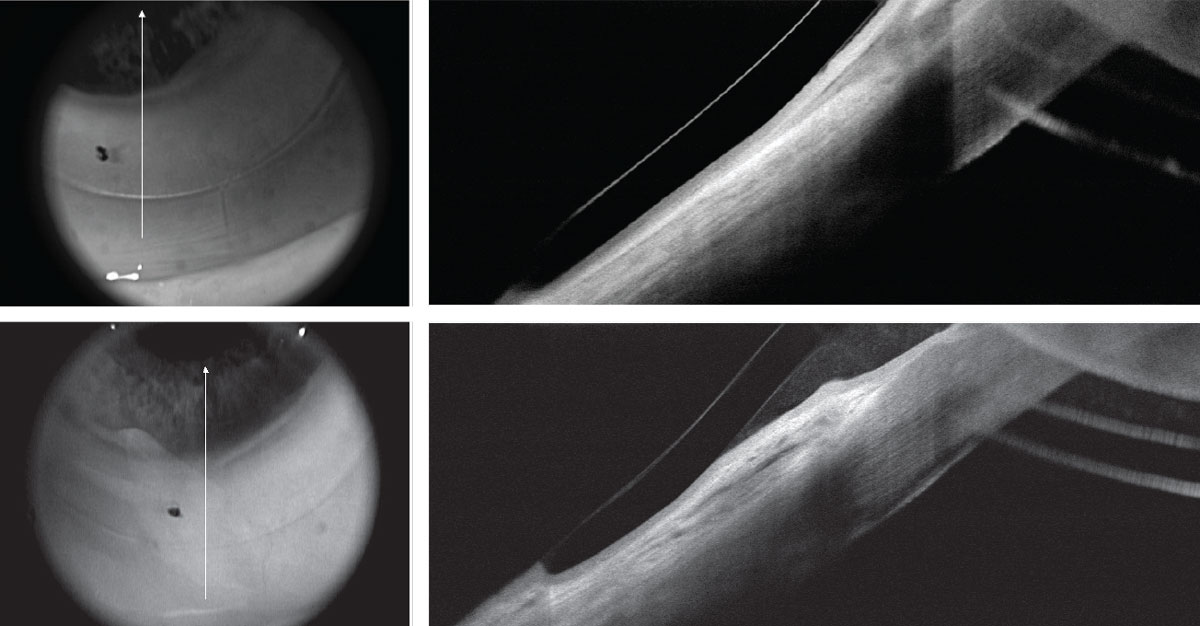 |
| Fig 10. (Top) Post-RK/LASIK OCT with EyePrint prosthetic device in place shows impingement of the inferior conjunctiva due to conjunctival redundancy and chalasis. (Bottom) Same area following chalasis surgery without loose conjunctival entrapment. Click image to enlarge. |
Corneo-scleral profile measurements are now becoming clinically available to anterior segment and contact lens professionals. These measurements have shed light on the complexity of the scleral surface shape and have led to the development of advanced scleral lens designs that better contour to the entire anterior ocular surface.10 New instruments, such as the Eaglet Eye Surface Profiler (Eaglet Eye) and the sMap 3D (Precision Ocular Metrology), and software developments like the CSP Pentacam software bring the measurement of corneal and scleral shape into the hands of eye care professionals.
The ability to take an ocular surface impression, as is possible with the EyePrint system (EyePrint Prosthetics) expands the ability to create a detailed model with precision that is unmatched. With these tools, we can now develop scleral lenses and scleral prosthetic devices that can contour even the most irregular ocular surfaces. That being said, conditions of the ocular surface, such as conjunctivochalasis, can continue to challenge ocular comfort and lens fitting success.11 In certain cases, surgical intervention is the best option to address the issues of chalasis and to allow patients to return to comfortable and effective contact lens wear.12
Advancements in imaging technologies have significantly improved our ability to both diagnose and manage corneal and other anterior segment diseases. Investing in such technologies provides a return of investment that goes far beyond the financial accounting—it results in better care for our patients.
Dr. Eiden is the president and medical director of North Suburban Vision Consultants in Illinois and the president and cofounder of the International Keratoconus Academy.
|
1. Belin MW, Asota IM, Ambrosio R Jr, Khachikian SS. What’s in a name, keratoconus, pellucid marginal degeneration and related thinning disorders. Am J Ophthalmol. 2011;152(2):157-62. 2. Pepose J. Wavefront aberrations in patients with keratoconus and pellucid marginal degeneration. Invest Ophthalmol Vis Sci. 2004;45(13):2893. 3. Kamiya K, Hirohara Y, Mihashi T, et al. Progression of pellucid marginal degeneration and higher-order wavefront aberration of the cornea. Jpn J Ophthalmol. 2003;47(5):523-5. 4. Gomes JA, Rapuano CJ, Belin MW, Ambrósio R Jr; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-69. 5. Kanellopoulos A, Asimellis G. OCT corneal epithelial topographic asymmetry as a sensitive diagnostic tool for early and advancing keratoconus. Clin Ophthalmol. 2014;8:2277-87. 6. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267-73. 7. Godefrooij DA, de Wit GA, Uiterwaal CS, et al. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169-72. 8. Bao F, Geraghty B, Wang Q, Elsheikh A. Role of corneal biomechanics in the diagnosis and management of keratoconus. In: Alió J, ed. Keratoconus: recent advances in diagnosis and treatment. Cham, Switzerland:Springer;2017;141-50. 9. Bykhovskaya Y, Margines B2, Rabinowitz YS. Genetics in Keratoconus: where are we? Eye Vis (Lond). 2016;3:16. 10. van der Worp E. A Guide to Scleral Lens Fitting, Version 2.0 [monograph online]. Forest Grove, OR: Pacific University; 2015. commons.pacificu.edu/mono/10. Accessed March 17, 2019. 11. Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998;43(3):225-32. 12. Marmalidou A, Kheirkhah A, Dana R. Conjunctivochalasis: a systematic review. Surv Ophthalmol. 2018;63(4):554-64. |


