Inflammation—we’ve all seen this quintessential part of the body’s defense network in action, whether due to a serious infection or simply a stubbed toe. The inflammatory process is an immunovascular response involving immune cells, blood vessels and molecular mediators designed to first eliminate the initial cause of cell injury, then remove any necrotic cells or damaged tissues and initiate tissue repair. Its effectiveness requires a delicate balance: insufficient inflammation can lead to progressive tissue destruction by the harmful stimulus (e.g., bacteria) and compromise the survival of the organism, while chronic inflammation can result in loss of tissue function, chronic pain and scarring.
In effect, the concept of inflammation as both a normal, protective physiologic process and as a pathologic damaging process embodies what’s known as the Goldilocks principle: too little and too much are bad; it must be “just right.” So, how can we as eye care practitioners achieve this equilibrium in an environment as complex as the eye?
| Table 1. Adverse Corticosteroid Effects | |
| Extended exposure to high-dose systemic corticosteroids produces: | |
| Weight gain | Hypercalcemia |
| Baldness | Anxiety |
| Truncal obesity | Acne |
| Impotence | Depression |
| Hump back | Hirsutism |
| Amenorrhea | Dry, brittle hair |
| Moon face | Hyperhidrosis |
| Psychosis | Skin discoloration |
Corticosteroids
Considered the “Swiss army knives” of inflammation control, corticosteroids act as palliative treatment for a host of inflammatory disorders (e.g., uveitis, episcleritis and scleritis) and adjunctive therapy for inflammation associated with injury and infection. Patients suffering from rheumatoid, arteritic, atopic and allergic diseases may also benefit from steroid therapy.
Two primary types of corticosteroids exist: ketones (prednisolone, dexamethasone, fluorometholone, medrysone and rimexolone) and esters (loteprednol). Ketone steroids depend upon liver metabolism to become inactive, while ester steroids do not; instead, they are inactivated locally by a single hydrolytic step. Hydrolysis, from Greek hydro- (“water”) and lysis (“separation”), means the cleavage of chemical bonds by the addition of water. Because the rapid cleavage of ester steroids produces inactive metabolites, these drugs are classified as “soft” steroids. Clinically, this results in a lower incidence of steroid glaucoma (10% less with use of loteprednol 0.5%) and a lower incidence of steroid cataract.
Ultimately, the therapeutic goal of corticosteroid use is resolution of the inflammatory response without any adverse ocular or systemic effects, steroid withdrawal symptoms or effect on normal production of endogenous glucocorticoids. As such, if the diagnosis, dosage or drug is incorrect, the use of these potent drugs can lead to consequences that can be both life- and sight-threatening.
Additionally, while topical steroids are effective to treat superficial or anterior segment inflammation, systemic treatment is necessary to treat diseases of the orbit or posterior segment, as well as arteritic conditions. Thus, as the optometrist’s prescriptive authority expands to include systemic drugs, one must understand both the physiology of the endogenous corticosteroids and pharmacology of their synthetic analogs in order to fully use these agents in a successful anti-inflammatory regimen. This understanding must then be applied when selecting the most effective product and dosing regimen to appropriately treat the patient's condition. The optometrist should also be able to establish clinical monitoring parameters to identify adverse or toxic reactions and significant drug-to-drug interactions with respect to corticosteroid therapy.
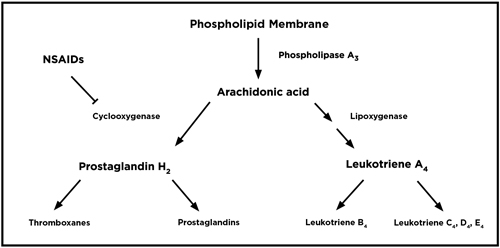 | |
| Fig. 1. The arachidonic cascade in inflammation. |
• Method of Action. Corticosteroids work at all points of the immune system to inhibit humoral (i.e., antibody production) and cell-mediated (i.e., late-phase cellular response) immune responses, as well as the production of phospholipase A, which leads to a reduction in the body’s major inflammatory cytokines, prostaglandins and leukotrienes (Figure 1). For example, in rheumatoid arthritis, the body’s immune system produces an abnormal form of the IgM antibody via plasma cells that attack normal IgG antibodies. This process eventually results in the inflammatory tissue damage that we see in rheumatoid disease as well as in ocular conditions like uveitis, scleritis and episcleritis. Steroids inhibit plasma cell production of IgM, thus inhibiting inflammation. Additionally, leukotriene production can attract T-lymphocytes that are responsible, in part, for late-phase chronic inflammation, so reducing these cytokines can limit chronic inflammation.
 | |
| Topical steroids are a mainstay therapy for inflammatory conditions like iritis. Photo: Christine W. Sindt, OD |
When stress or other neural stimulation is placed on the hypothalamus, cortocotropin-releasing factor (CRF) is released, which acts on the anterior pituitary gland to stimulate the release of adrenocorticotropic hormone (ACTH). ACTH then acts as an agonist on cells of the adrenal cortex, causing the production of the glucocorticoid cortisol. As blood cortisol levels rise, they inhibit production of CRF by the hypothalamus, thus inhibiting excessive cortisol production. This is called the negative feedback loop.
Both endogenous systemic cortisol and exogenously administered synthetic glucocorticoids will produce adrenal glucocorticoid suppression; thus, use of corticosteroids for more than a few weeks can lead to adrenal suppression and adrenal atrophy (i.e., Addison’s disease). Long-term use of systemic corticosteroids can also lead to Cushing’s syndrome (Table 1). Topical ophthalmic steroids, however, do not produce these adverse effects.
• Glucogorticoid vs. Mineralocorticoid Effect. Cortisol (hydrocortisone), the primary glucocorticoid produced by the adrenal cortex, is responsible for carbohydrate metabolism. Its overproduction or pharmacologic use may result in hyperglycemia or glucose intolerance. Aldosterone is the major mineralocorticoid, and plays a role in the retention of salt and water to maintain proper fluid/electrolyte balance and blood pressure. Synthetic corticosteroids vary in their relative balance of mineralocorticoid and glucocorticoid effects; however, because all synthetic glucocorticoids can produce some degree of water and salt retention and hyperglycemia, they should be used cautiously in cardiovascular and diabetic patients.
| Table 2. Relative Steroid Potency | |
| Hydrocortisone 20mg is equivalent in potency to: | |
| Betamthasone | 0.6mg |
| Dexamethasone | 0.75mg |
| Paramethasone | 2mg |
| Methylprednisolone | 4mg |
| Triamcinolone | 4mg |
| Prednisone | 5mg |
| Prednisolone | 5mg |
| Cortisone | 25mg |
Most of the common oral synthetic corticosteroids have similar glucocorticoid/mineralocorticoid activity, with the main difference being potency. Dosage is calculated based on steroid equivalents, with 20mg of cortisol acting as the baseline dose to which all other glucocorticoid potency is compared (Table 2).
• Ocular Use. The ocular properties of corticosteroids are different in some aspect from their systemic counterparts. The ocular version must be in an active form, since it is applied topically and will not undergo hepatic metabolism before reaching site of action. Additionally, it must be capable of penetrating corneal tissues and must possess adequate potency to significantly reduce the local inflammatory response.
Table 3 lists many of the most common ocular conditions that respond to topical and, if necessary, systemic corticosteroid therapy. These agents are particularly effective in the palliative treatment of allergic conjunctivitis or keratoconjunctivitis, and any noninfectious inflammatory condition in the eye because they are able to affect the immune system at so many levels (i.e., inhibition of cytokines, antibody production and T-cell and eosinophil migration).
If the issue is a result of bacterial infection, which can be adequately treated with antibiotic therapy, adjunctive corticosteroid therapy may be used. Note that the concomitant use of corticosteroids within an antiviral agent in the treatment of herpes simplex keratitis is more controversial because their use is limited to disciform keratitis and contraindicated for active dendritic disease in particular.1 Additionally, corticosteroids should not be used in fungal infections, since the steroid may worsen infection.
| Table 3. Ocular Indications for Corticosteroids Allergic conjunctivitis/blepharitis Superior punctate keratitis GPC/VKC/AKC Posterior uveritis Contact dermatitis Juvenile xanthogranuloma Immune graft rejection Sympathetic ophthalmia Optic neuritis Ocular burns Cranial arteritis Episcleritis/scleritis Graves disease Rosacea keratitis Phlectenular keratoconjunctivitis Ocular pemphigus Orbital pseudotumor Iritis/iridocyclitis Marginal corneal ulcers Trabeculitis Inflammatory complications Intersitital keratitis of herpetic disease Retinal vasculitis Epidemic keratoconjunctivitis Infiltrative keratitis |
When inflammation is due to allergens, the offending stimulus should first be removed if possible. Mast-cell inhibitors or antihistamines can be used to prevent further reaction.
• Sizing Up the Corticosteroid Options. When choosing a topical steroid, consider which one has the lowest effective dosage, longest dosing interval and the shortest duration of therapy to prevent adverse effects and allow for discontinuation without withdrawal symptoms or flare-up of the disease. However, overall the specific corticosteroid product and dosage should be chosen based on the severity of inflammation (Table 4).
Prednisolone acetate 1% is the most active ocular corticosteroid, and should be the drug of choice when maximal anti-inflammatory effect is required: a dosage of one drop every minute for five minutes each hour has been shown to decrease ocular inflammation by 72%, as compared to a decrease of 51% with hourly dosing and 11% with doses every four hours.2 This suggests that cumulative dose increases activity of the anti-inflammatory effect of the agent when applied topically.
One of the newer high-potency topical steroids is difluprednate 0.05% (Durezol, Alcon), which has been demonstrated to have an efficacy comparable to that of prednisolone acetate 1% in anterior uveitis.3 One benefit of Durezol is that it does not need to be shaken, as it is an emulsion; however, prednisolone acetate is available in a much lower-cost generic formulation. Both drugs raise intraocular pressure (IOP) and increase the risk of cataract formation.
• Steroid Complications. In general, due to high receptor affinity and rapid inactivation, the likelihood of steroid-linked cataract formation and glaucoma is significantly decreased. Steroid response to loteprednol is less than 3%, according to an FDA comparison of loteprednol 0.2% (Alrex) and 0.5% (Lotemax, B+L) to prednisolone acetate 1%. The study measured the incidence of an increase in IOP>10mm Hg over a 28-day period; prednisolone acetate raised IOP in 7% of tested patients.4
The use of corticosteroids has been linked to cataract formation in patients with rheumatoid arthritis. A study in 1961 at the National Institutes of Health (NIH) found 17 of 47 patients with rheumatoid arthritis who received prednisone for more than one year developed cataracts, compared with none in the 19 patients who did not receive the steroids.5 Specifically, posterior subcapsular cataracts (PSC) were observed in 36% of patients treated with steroids for one to four years and in 69% of patients treated for more than four years.6 With respect to different dosages of prednisone, 23% of patients treated with a dose 10mg to 15mg per day and 75% of those receiving prednisone equivalent to more than 15mg per day developed cataracts. None of the six patients receiving less than 10mg per day of prednisone and none of the nine patients receiving steroids for less than one year developed cataracts.
PSCs resulting from topical use are similar in presentation to those caused by systemic drugs. Most reports of PSC are secondary to topical ocular corticosteroids that have been administered for more than six months.
Corticosteroids have also been shown to increase IOP. A study evaluating 14 known corticosteroid responders identified IOP increases of 4.4mm Hg to 8.1mm Hg with fluorometholone 0.25% suspension compared to 8.1mm Hg to 11.6mm Hg with dexamethasone sodium phosphate solution 0.1%.7
| Table 4. Relative Topical Ocular Anti-Inflammatory Steroid Efficacy (When Corneal Epithelium is Intact) | |
| Drug | Decrease in Inflammation |
| Prednisone acetate 1% | 51% |
| Dexamethasone alcohol 0.1% | 40% |
| Fluromethalone alcohol 0.1% | 31% |
| Prednisolone sodium phosphate 1% | 28% |
| Dexamethasone sodium phosphate | 19% |
| Dexamethasone sodium phosphate 0.05% oint. | 13% |
A separate study compared the effects of betamethasone 0.1% combined with sulfacetamide 10% on IOP in three groups of patients treated with a single drop QID for up to two months. Researchers observed a mean IOP increase from 16.9mm Hg to 32.1mm Hg in the 44 patients with primary open angle glaucoma and a mean increase from 17.1mm Hg to 28.3mm Hg in the 32 glaucoma suspects. The 30 normal subjects had a mean pressure increase from 13.6mm Hg to 18.2mm Hg.8 A second study completed later indicated that IOP increase as a reaction to corticosteroids may be genetically determined; specifically, it is possible that primary open angle glaucoma patients are homozygous carriers of a glaucoma gene, non-glaucoma responders are heterozygous for the glaucoma gene and non-responders are homozygous for the normal (i.e., non-glaucoma) gene.9
NSAIDs
Nonsteroidal anti-inflammatory drugs (NSAIDs) also play a very important role in the management of ocular disease, despite having an anti-inflammatory efficacy below that of the corticosteroids. Both the topical (Table 5) and systemic NSAIDs are used to manage mild to moderate pain and are frequently combined with opiates like codeine, hydrocodone and oxycodone to enhance their analgesic effect. Topical ocular NSAIDs in particular are used to manage postoperative pain, miosis and cystoid macular edema (CME). It should be noted, however, that all NSAIDs have some degree of potential to inhibit the beneficial antiplatelet activity of aspirin.
Nonselective NSAIDs work by inhibiting all forms of the enzyme cyclooxygenase (COX), which is responsible for the formation of prostanoids (i.e., prostaglandins, thromboxanes and prostacyclins) that mediate inflammation, anaphylaxis and vasoconstriction (Figure 1). Prostaglandins in particular play a role in the direct stimulation of pain receptors (nociceptors) and vasodilation (hyperemia), but do not affect the lipoxygenase/leukotriene pathway.
| Table 5. Currently Available Topical Ocular NSAIDs Acular (ketorolac 0.5%, Allergan) Acular LS (ketotolac 0.4%, Allergan) Ocufen (Flurbiprofen 0.03%, Allergan) Diclofenac 0.1% (generic) Voltaren (diclofenac 0.1%, Novartis) Prolensa (bromfenac 0.07%, B+L) Nevanac (nepafenac 0.1%, Alcon) Ilevro (nepafenac 0.3%, Alcon) |
Two forms of cyclooxygenase exist: COX-1 is a constitutive enzyme that is continuously produced and is responsible for the production of prostaglandins necessary for normal physiologic functions, including fabrication of the stomach’s protective coating (i.e., the gastric mucosal barrier), adequate renal blood flow and normal blood clotting. Inhibition of COX-1 can lead to gastric irritation, peptic ulcers, gastrointestinal bleeding and blood clotting disorders.
COX-2 is an inductive enzyme that is produced in response to tissue injury. Inhibition of COX-2 can lead to gastrointestinal irritation and ulceration; drugs specifically designed to impede COX-2 can also increase the risk of heart attack and stroke.
• Antipyretic Effect. NSAIDs are also known to reduce fever. When the body’s healthy state is compromised as a result of malignancy, infection or the introduction of certain chemicals, it raises its internal temperature to increase overall metabolism, enhancing its ability to fight invaders. This elevated temperature may also inhibit bacterial growth, because pathogenic bacteria typically only grow within narrow temperature ranges.
This process is triggered by the release of interleukin 1 cytokines, which stimulates the synthesis of prostaglandins E1 and prostaglandin F2. The prostaglandins then reset the hypothalamic thermostat to a level above the normal 37°C to raise the body’s temperature. The NSAIDs are effective at low doses in reducing this elevated temperature, but have no such effect on normal or subnormal temperature.
• Hemostasis. Arachidonic acid is a precursor in the synthesis of the prostaglandin analogs prostacyclin and thromboxane A2. Thromboxane initiates platelet aggregation, while prostacyclin antagonizes aggregation. Under normal circumstances, the two analogs are physiological antagonists, and the platelets do not aggregate. The action of NSAIDs on the prostaglandin endoperoxide synthetase (or cyclooxygenase) causes inhibition of platelet aggregation, thus prolonging bleeding time—an effect that can either be therapeutic or cause an adverse reaction.
• Off-Label Use. Some evidence for the efficacy of topical NSAIDs in the management of retinal edema associated with epiretinal membranes, diabetic macular edema and retinal vein occlusions does exist; however, more information is needed before their use can be recommended.
Dr. Onofrey is a clinical professor and executive director of continuing education programs at the University of Houston. He is also an internationally recognized lecturer on pharmaceutical agents and ocular disease management.
1. Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994 Dec;101(12):1871-82.
2. Leibowitz HM. Management of inflammation in the cornea and conjunctiva. Ophthalmology. 1980 Aug;87(8):753-8.
3. Foster CS, Davanzo R, Flynn TE, et al. Durezol (Difluprednate 0.05% ophthalmic emulsion) compared with Pred Forte 1% Ophthalmic suspension in the treatment of endogenous anterior uveitis. J. Oc Pharmacol Ther. 2010 Oct 26 (5). 475-83.
4. Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004 Sep;138(3):444-57.
5. Oglesby RB, Black RL, von Sallmann L, Bunim JJ. Cataracts in rheumatoid arthritis patients treated with corticosteroids. Arch Ophthalmol. 1961 Oct:66:519-23.
6. Black RL, Oglesby RB, von Sallmann L, Bunim JJ. Posterior Subcapsular Cataracts Induced by Corticosteroids in Patients with Rheumatoid Arthritis. JAMA 1960:174(2):166-71.
7. Kass M, Cheetham J, Duzman E, Burke PJ. The ocular hypertensive effect of 0.25% fluorometholone in corticosteroid responders. Am J Opthalmol. 1986 Aug 15:102(2):159-63.
8. Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Opthalmol 1963:70(4):500-7.
9. Becker B. The effect of topical corticosteroids in secondary glaucomas. Arch Opthalmol. 1964:72(6):769-71.


