The continuing expansion of eye care has shown that, as practitioners, we are capable of dealing with myriad eye disorders. Although we can manage various diseases with the help of drugs, we still see chronic eye conditions that continue to affect our aging population with increasing frequency. Some view our current health care system as less “health care” and more “disease management.” But, over the past two decades, research on ocular health has shown that nutrition has a significant effect on many eye disorders, and eye care practitioners are in a perfect position to embrace this treatment protocol.
Regulation Standards
Should we approach nutrition with caution? Without a doubt! Much the same way we approach pharmacological agents with caution, nutrition must be recommended with scientific support and logical application. While nutritional products are not controlled with the same protocol as pharmaceutical agents, they are very well regulated. Take a look at the different levels of approvals of drugs, foods and supplements (see “Regulation of Products by the U.S. Food and Drug Administration,” below. As you can see, the supplements are closely related to food and only differ from drugs in the pre- vs. post-market approval.
The Dietary Safety and Health Education Act (DSHEA, 1994) defines nutritional products and what controls the FDA has over them. It is true that dietary supplement products do not have to undergo the same pre-market approval process as that required for drugs; however, pre-market approval does not guarantee safety. Pharmaceutical products in the past, have been granted FDA approval, only to later be removed from the market for safety reasons. In addition, dietary supplement manufacturers adhere to a variety of other requirements to help assure product safety. For example, if serious adverse events are reported, supplement manufacturers must notify the FDA within 15 days. This is a requirement comparable to prescription and some over-the-counter drugs, but not conventional foods.
Regulation of Products by the U.S. Food and Drug Administration
FDA-regulated products
New Drug Application
Pre-market notification
Labeling
Mandatory adverse
event reporting
CGMPs
Facility registration
Advertising (FTC or FDA)
Foods
X
X
X
X
X
Dietary supplements
X
X
X
X
X
X
Drugs
X
X
X
X
X
X
Courtesy: The Council for Responsible Nutrition
Efficacy of Nutritional Supplements
The gold standard for establishing the efficacy of pharmacological agents is the randomized controlled trial (RCT). However, this is poorly suited for the evaluation of nutritional effects for several reasons. Chronic diseases have long latency and multi-factorial causation, so it is difficult to determine exactly when a disease process begins and how long it has been developing.
For the most part, nutrients interact with one another, whereas drugs are designed to work solo. All foods contain a variety of nutritional ingredients that are designed to work synergistically. And lastly, we would like there to be only one target tissue with drug therapy, but side effects do occur. While nutrients act in modest beneficial ways in virtually every body system, drugs act potentially on single targets (the RCT is designed for single outcomes).
Many studies performed on nutrients are considered “observational” studies. There are, however, limits to observational studies as well. In an observational study, there is the absence of a no-exposure group. (One cannot require subjects in a study to not take a specific nutritional product for an extended period of time; there can only be different levels of intake.) Uncertainty in quantitative measures of food intake due to inaccurate reporting and recall bias is often the case. Either the subject fails to accurately record the type and amount of food eaten, or they forget and attempt to recall at a later time. Some studies actually record food intake at the beginning and end of a particular study and assume that the subjects are eating similarly throughout the entire duration of the study!
There is also uncertainty in the translation of food to nutrient intake. Inaccurate food composition tables, natural variability, processing effects and poor correspondence between nutrients in certain foods are common problems in determining the actual nutrient intake.
This is not to say that there are no unscrupulous manufacturers and suppliers in the nutritional product industry. There may be misleading ads for weight loss, miracle cures and unfounded effects of any product, but these claims are under the regulation of the Federal Trade Commission. It is incumbent upon the practitioner to research the companies that make supplements (including their advisory board members as well as the ingredients in their products) and confirm that they have legitimate science behind their formulations.
In addition, practitioners must decipher between the myriad multi-level marketing (MLM) programs in the marketplace. While there are some good products that are marketed this way, MLM tends to promote the advantages of generating income over the concept of good health, so the MLM does not necessarily fit into the professional image of the eye care practitioner.
The Nutritional Link
Follow These Simple Rules in Nutritional Supplementation
•
Balance. The body uses nutrition to fuel and heal itself, but it will
not always respond to excessive amounts of one nutrient. Overuse of one
ingredient can create a deficiency in another.
• There is no “single bullet.” One specific nutrient will not cure any one particular condition.
•
Consistency. It matters not what you do once in a while, but what you
do every day. If you stray from your diet and eat too much of the wrong
foods one day, don’t worry! Just get back to your sensible diet the
next day.
• Moderation. If 10mg of a supplement is effective, it
does not mean that 100mg is 10 times more effective! This is especially
true with herbal remedies, which can create more severe reactions than
vitamin supplements.
We started as a “drugless” profession more than 100 years ago. And, while we have progressed in the depth and breadth of our scope of practice, we should not forget our roots. The body is a great healer and might simply require additional “support” to re-balance itself. I do not mean to take away from the power and necessity of our medical treatments; the fact remains that an acute eye condition requires immediate attention. Nutrition will not be appropriate for this type of treatment, but it is very appropriate for the support of human health and should be a part of the primary care practitioner’s armamentarium of products for eye health. A classic example is dry eye syndrome.
While this is certainly a significant issue for millions of patients, it is usually a slow-developing, chronic condition that is rarely sight-threatening. Over the years, we have recommended over-the-counter drops and other palliative treatments, but nutrition has found its way into this treatment plan. Why? Because it works! Oral nutrition supplementation is becoming well recognized in the medical and eye care professions as an effective way to alleviate the symptoms of dry eye syndrome.
Considering some specific eye diseases, we can find several studies with a nutritional link. The “gold standard” study is the Age-Related Eye Disease Study (AREDS), which was published in 2001.1 While it was a positive step toward using nutrition to slow the progression of AMD, the science that was used to develop the formula was from the mid-1980s. We now realize that there are several other nutrients involved with macular health, and thus AREDS 2, with a more elaborate formula, is now under way.
A 1998 study in Ophthalmology indicates that a vitamin A deficiency (more prevalent in developing countries) can be a predisposing factor in microbial keratitis.2 Another study on retinitis pigmentosa suggests that vitamin A can have a beneficial effect on this condition as well.3 Several studies have also shown that vitamin A is a factor in the health of epithelial tissue (such as the corneal epithelium). Vitamin C, magnesium and several other nutrients have been shown to lower eye pressure.1 A nurse’s study in 2009 showed a reduced risk of AMD with an increase in certain B vitamins.4
Furthermore, most eye care practitioners are aware of the influence of omega-3 and omega-6 essential fatty acids (EFAs) on cellular function, especially when it comes to dry eye. This is particularly significant in the eye and nervous system because the retinal cells contain the highest level of docosahexaenoic acid (DHA) in the body. The EFAs are derived from ingesting fish oils; they support cellular energy levels and are critical in cellular function.5 The FDA has regulatory responsibility over the import of fish oils, and an independent evaluation by Consumer Labs ( www.consumerlab.com) of more than 50 brands of fish oil has shown that none are contaminated with any toxic levels of mercury or PCB, which can be poisonous to humans. Therefore, taking fish oil capsules is a safe way to get the EFAs we need on a daily basis.
Integrative Medicine
Practitioners must realize that recommending nutrition and dietary supplements can be a safe and effective tool in the prevention and treatment of chronic eye disease. (When recommending products, be sure to check out the manufacturer. Reputable companies have their board of directors, as well as their product’s ingredients, openly listed on their websites.) It is up to us to know which supplements to recommend because consumers can easily get confused. So, taking some extra time to learn about nutrition and supplementation will advance your understanding of your patients’ needs and potential resolutions to their eye care concerns.
Talk to your patients. The benefits of nutritional counseling are many. The practitioner is addressing the patient’s main problem. Since we are now becoming more aware of the link between chronic disease and eye disease, we are using a more universal approach. In patient education, the practitioner gains an insight into the patient’s overall health. Nutritional counseling will bring patients back to the office frequently, and it is better that they receive this information from you, rather than the health food store clerk or the hairdresser. This enhances your reputation as a primary care provider.
Supplements are here to stay. More than half of the U.S. adult population uses dietary supplements, and most supplement users take them every day. Supplements can fill gaps in nutrient intake, and the potential health benefits are substantial. Integrative medicine takes account of the whole person, including all aspects of lifestyle. It emphasizes the therapeutic relationship and makes use of both appropriate conventional and alternative therapies. It is a philosophy that neither rejects conventional medicine nor accepts alternative therapies uncritically and uses natural, effective, less-invasive interventions whenever possible. I hope that eye care practitioners are ready to embrace this healing approach.
Dr. Anshel is the president of the
Optometric Nutrition Society and maintains a private practice in
Carlsbad, Calif. The second edition of his book, “Smart Medicine for
Your Eyes,” is out now.
1. Engin KN, Engin G, Kucuksahin H, et al. Clinical evaluation of the neuroprotective effect of alpha-tocopherol against glaucomatous damage Eur J Ophthalmol. 2007 Jul-Aug;17(4):528-33.
2. Kunimoto DY, Sharma S, Reddy MK, et al. Microbial keratitis in children. Ophthalmology. 1998 Feb;105(2):252-7.
3. Berson EL, Rosner B, Sandberg MA, Dryja TP. Ocular findings in patients with autosomal dominant retinitis pigmentosa and a rhodopsin gene defect (Pro-23-His). Arch Ophthalmol. 1991 Jan;109(1):92-101.
4. Christen W, Glynn R, Chew E, et al. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med. 2009 Feb 23;169(4):335-41.
5. Effects of omega-3 fatty acids on cardiovascular disease. Available at: www.ahcpr.gov/clinic/epcsums/o3cardsum.htm/dec.2004. (Accessed January 2010).
RX TREATMENT
The importance of proper nutrition is undeniable. An estimated two billion people worldwide suffer from some form of micronutrient deficiency, according to the Food and Agricultural Organization of the United Nations.1 In particular, iodine deficiency is the single greatest preventable cause of mental retardation.2 Malnutrition is linked to the deaths of almost six million children each year worldwide.3 Although malnutrition is highly prevalent in developing countries in South Asia and Africa, its related mortality and morbidity is fortunately uncommon in the United States.
Despite the significant impact of malnutrition globally, ocular disease directly stemming from micronutrient deficiency seems to have very low prevalence in the United States. As an example, most eye care practitioners in the United States probably have never had a patient with vitamin A deficiency with conjunctival xerosis (Bitot’s spots) and nyctalopia. Nor have most practitioners diagnosed tobacco-alcohol amblyopia, where nutritional deficiency from tobacco and alcohol abuse leads to papillomacular bundle damage.4
Despite the paucity of eye morbidity linked to malnutrition here, strategic nutritional supplementation may help prevent or mitigate specific eye conditions related to aging and genetic predisposition. Indeed, reasonable evidence supports particular nutritional supplements for macular degeneration. The Age-Related Eye Disease Study (AREDS) by the National Eye Institute found that patients at high risk of developing advanced stages of AMD lowered their risk by about 25% when treated with a high-dose combination of vitamin C, vitamin E, beta-carotene and zinc.5 Elsewhere in eye care, scientific evidence backs the use of certain nutritional supplements. Yet, there are also large vacancies in this type of information.
Evidence for Dietary Supplements
There is a conspicuous void in evidence-based support for many vitamins, minerals, herbs and other botanicals, amino acids, various extracts and combinations. This is because these substances qualify as “dietary supplements” according to the Dietary Supplement Health and Education Act (DSHEA) of 1994 and are exempt from the scrutiny endured by prescriptive medicine. Congress passed DSHEA following heavy lobbying by the supplement industry. As a result, supplement manufacturers are not required to submit safety or efficacy data to the FDA before marketing dietary supplements. In effect, the FDA assumes the burden of proving a dietary supplement is harmful rather than the manufacturer proving that the supplement is safe.6
DSHEA allows the manufacturer to imply benefits on the “structure and function” or general “well-being” of the body, provided that the following disclaimer is given: “These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.”
As an example, a manufacturer of a bilberry supplement can label the bottle to say the product reduces eyestrain and supports vision, even though there is strong evidence against the validity of such claims.7 Without requirements upon dietary supplement manufacturers to demonstrate safety and efficacy, large-scale studies such as AREDS will likely be few and sparse.
The Rigors of New Drug Development
Unlike dietary supplements, the Federal Food, Drug and Cosmetic (FD&C) Act requires all new drugs to demonstrate safety and efficacy before they are marketed in the United States.8 The drug development and approval process is expensive and time consuming. And, the cost to develop a new drug—including studies conducted after receiving regulatory approval—average $897 million, according to an analysis by the Tufts Center for the Study of Drug Development.9 On average, it took 6.3 years of development time after investigational new drug applications were submitted for drugs that were approved between 1994 and 2002.10
Robert Meyer, M.D., a representative of the FDA, has provided insightful testimony on the new drug approval process.11 “The single most important public health provision in these statutes (the FD&C Act) is the requirement that a person wishing to sell to the public a product to prevent, cure or mitigate illness or injury must first prove that such product is safe, and actually does what the vendor claims it does. This statutory provision affords patients the most effective protection against untested and unproven products,” he says. “The evidence of safety and effectiveness usually is obtained through controlled clinical trials. The disciplined, systematic, scientific conduct of such trials is the most effective and certain means of obtaining the data that document safety and efficacy of a drug and how to use the new product so that it will have the most beneficial effect.”11
Despite the regulatory scrutiny given to prescriptive drugs, FDA approval hardly conveys immunity to recalls, market withdrawals and safety alerts. There are frequent instances where additional information about a drug—both good and bad—becomes available after commercialization. Over the past few years, high-profile recalls for Vioxx (rofecoxib, Merck), Rezulin (troglitazone, Parke-Davis) and Baycol (cerivastatin, Bayer A.G.), may have shaken consumer confidence in prescriptive drugs. In the same manner that news media tend to cover airplane crashes over safe landings, the availability bias of drug recalls may have led consumers to seek holistic and natural treatments, including nutritional supplementation. Yet, these alternative and complementary treatments sometimes raise worrisome issues of their own.
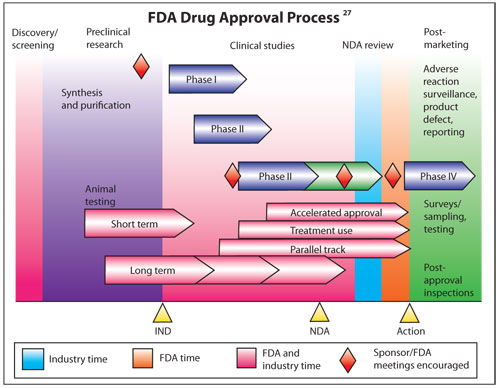
Unlike dietary supplements, new
drugs must earn New Drug Application (NDA) approval prior to U.S.
commercialization. This process to provide safety and efficacy data is
expensive and time consuming.
“Natural” vs. “Safe and Effective”
A frequent, but mistaken, concept is that dietary supplements are always better in some regard just because they contain natural substances. To dispel that notion, consider that cyanide occurs naturally in cassava plants, and ergot poisoning can naturally occur from eating rye. You can even die from drinking too much water from hyponatremia, a condition where electrolyte concentration in the body is diluted to dangerously low levels. Just because something is natural—such as cassava, rye or water—doesn’t mean it is necessarily safe, especially under the wrong circumstances.
Consumers and practitioners are frequently attracted to dietary supplements because of a perceived lack of side effects. In reality, however, safety and efficacy information on dietary supplements does not always exist. One commonly cited example in which safety problems may have come to light with careful study before commercialization is with ephedra-containing dietary supplements. The FDA banned the sale of ephedra-containing supplements in 2004 after compiling 19,000 adverse event reports and 155 deaths involving ephedra.12 That ruling was upheld in 2006 when challenged in a federal appeals court, despite fierce opposition by dietary supplement manufacturers.13 The ephedra experience has prompted critics to call for greater regulation of dietary supplements, similar to what prescription medicines go through, so that there is reasonable characterization of safety and efficacy prior to commercialization.
Although safety and efficacy data are often lacking for dietary supplements, this type of information is reasonably established for all prescriptive medicines and is presented in each drug’s prescribing information (PI) package insert. Consequently, for evidenced-based practitioners, prescriptive drug therapy offers greater outcomes predictability over many dietary supplements.
A Permissive Environment to Promote Without Evidence
According to the Federal Trade Commission (FTC), MLM is a way of selling goods where if you sign up as a distributor, you’re promised commissions for both your sales and those of others you recruit to serve as distributors.14 The FTC cautions against joining MLMs, particularly those that offer to pay commissions for recruiting new members, as that may constitute an illegal “pyramid” scheme in many states.
Yet, there are MLMs targeting eye care practitioners with promotions such as this:
“What if I told you that you could receive a check each month for doing something you’re already doing ... pulling out your Rx pad and recommending an OTC supplement for AMD … Just one patient a day will generate $25,740 in additional net income in your first year and $85,470 in year two!”15
Aspiring for financial success is virtuous in a capitalistic society. But, heavy emphasis on profiting off patients raises ethical concerns—particularly if good scientific evidence is ignored in favor of promotion through anecdote and case inferences.
Without calling them out, certain dietary supplement marketers employ questionable business tactics—including promoting studies with design flaws, such as questionable controls, inadequate sample sizes or improper masking of bias. These attempts to control perception regretfully lead some of our patients to believe that rigorous evidence supports the promoted nutritional supplement.
Treating “Pink Eye” With a Fruit Juice? Bananas!
Type into Google the terms “vitamins for pink eye,” and your search results will bring up some interesting web pages. One of them recommends an exclusive fruit diet as a treatment option for conjunctivitis.16
Needless to say, this type of nutritional treatment yields no support in the peer-reviewed literature. Most cases of bacterial conjunctivitis resolve by themselves without external intervention.17 Therefore, it is understandable how a patient with simple bacterial conjunctivitis could mistakenly attribute resolution to fruit juice treatment. This would be analogous to the rooster taking credit for the sun rising each morning.
Yet another website, run by an eye care practitioner, touts that herbs, such as burdock, forsythia, goldenseal and echinacea may help ease conjunctivitis.18 The author continues, stating that eating yogurt and applying yogurt compresses are cures for conjunctivitis. It should come as no surprise that there is little scientific basis for the aforementioned treatments. By comparison, there is ample support behind topical antibiotics for acute bacterial conjunctivitis.19 Aside from speeding up the resolution of bacterial conjunctivitis, antibiotics provide prophylaxis against spreading the infection to the fellow eye and to other people.
Colon Cleansing for Allergies?
One website suggests that allergy can be managed with supplements such as vitamin C, bioflavonoids, vitamins A, E and zinc.20 However use of most of these supplements is unsupported by a study that found that supplementation with antioxidants does not cause a change in immune responses, serum AC or plasma F(2)-isoprostanes.21
The same site also recommends cayenne pepper, garlic and even colon cleansing for allergies. Yet, since 1997, the FDA has issued at least seven warning letters related to colon cleansing, a procedure that is regarded as quackery by the National Council Against Health Fraud.22
At present, there is little basis for a reasonable practitioner to consider the aforementioned nutritional interventions for allergy. Especially with established safety and efficacy for topical ocular treatment—including antihistamine, mast-cell stabilizers and corticosteroids—the eye care professional has ample resources to safely and effectively manage the majority of ocular allergy presentations.
Snake Oil for Inflammatory Dry Eye?
To the layperson, fish oil may not sound that much different than snake oil. There is, however, reasonable evidence supporting the ingestion of fish oil for inflammatory dry eye. Omega-3 fatty acids in fish oil—particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—appear to reduce inflammation, in part by modulating eicosanoid synthesis. Key evidence for the benefits of omega-3 fatty acids for dry eye came from a study with 32,470 women between the ages of 45 and 84 enrolled in the Women’s Health Study.23 A food-frequency questionnaire administered in the study correlated women who consumed the highest amounts of omega-3 fatty acids, mainly from tuna, with the lowest rate of dry eyes.
There are concerns over potential contamination of fish oil supplements with methyl mercury, PCBs and organic pesticides. Pregnant women and children may be particularly susceptible to these contaminants. To address this concern, some manufacturers of fish oil supplements guarantee their products to be free of these contaminants. Additionally, in 2007, the FDA issued a final rule establishing regulations to require current good manufacturing practices (CGMPs) for dietary supplements. While the CGMP is now in effect, supplement manufacturers with less than 20 employees still have until June 2010 to comply with the regulations to assure a supplement’s purity, strength and composition.24
So, nutritional supplementation for inflammatory dry eye holds promise for becoming part of a standard treatment plan. But, with the many dry eye treatments available today, where might omega-3 fatty acids fit into the treatment regimen? For guidance, we can look toward expert consensus. The International Task Force (ITF) of dry eye specialists, often referred to as the Delphi Panel, came up with consensus guidelines for diagnosis and therapy of dysfunctional tear syndrome in 2004.25 The International Dry Eye Workshop Subcommittee (DEWS) later reviewed the Delphi approach and modified it.26 For mild dry eye disease (level 1), DEWS recommends education and counseling, environmental management, elimination of offending systemic medications and preserved tear substitutes and allergy eye drops. For moderate dry eye disease (level 2), DEWS recommends that if the level 1 treatments are inadequate, add unpreserved artificial tear substitutes, steroids, cyclosporine A, secretagogues and nutritional supplements (i.e., omega-3 fatty acids). Therefore, practitioners should consider omega-3 supplementation at least for moderate dry eye disease.
The Repercussions of Unsubstantiated Nutritional Treatment
A pill—whether a prescription drug or nutritional supplement—won’t cure a lifetime of smoking, excessive drinking, over-eating or lack of exercise. That said, prescription drugs play an important role in eye care. But, nutritional supplements are growing in importance, although data for their safety and efficacy is often missing. Because the FDA does not require efficacy or safety data for dietary supplements, a permissive environment exists for their manufacturers to make unsubstantiated claims. The lack of regulatory scrutiny of dietary supplements, unlike with prescriptive drugs, has attracted a plethora of business operations that overlook evidence-based methods. The danger of supplements to our patients is unneeded cost and delaying proper treatment. As practitioners, we must demand safety and efficacy for dietary supplements.
It is regretful that some dietary supplement companies are diverting practitioners into selling their unsubstantiated products through multi-level marketing schemes and other profit-centric tactics. The risk of eye care practitioners embracing questionable nutritional treatment, aside from serious patient care concerns, is that it reduces the credibility of our profession and damages our future strides into therapeutic treatment.
Dr. Chou serves on the editorial board for RCCL. He received commendation from the Federal Trade Commission for his detailed exposé of the now-defunct publisher of “Hidden Secrets to Better Vision,” a book that recommended dubious eye treatment.
1. Diouf J. World summit for social development world summit for social development. Available at: www.un.org/documents/ga/conf166/una/950307063316.htm. (Accessed December 2009).
2. Iodine deficiency–way to go yet. Lancet. 2008 Sept 13;372(9642):888.
3. Malnutrition Is a Major Contributor to Child Deaths. Available at: www.prb.org/Publications/Datasheets/2007/2007WorldPopulationDataSheet.aspx. (Accessed December 2009).
4. Behbehani R, Sergott RC, Savino PJ. Tobacco-alcohol amblyopia: a maculopathy? Br J Ophthalmol. 2005 Nov;89(11):1543-4.
5. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36.
6. Collins N, Tighe AP, Brunton SA, et al. Differences between dietary supplement and prescription drug omega-3 fatty acid formulations: a legislative and regulatory perspective. J Am Coll Nutr. 2008 Dec;27(6):659-6.
7. Canter PH, Ernst E. Anthocyanosides of vaccinium myrtillus (bilberry) for night vision – a systematic review of placebo-controlled trials. Surv Ophthalmol. 2004 Jan-Feb:49(1):38-50.
8. FDA. Federal Food, Drug and Cosmetic Act (FD&C Act). Available at: www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/default.htm. (Accessed December 2009).
9. Total cost to develop a new prescription drug, including cost of post–approval research, is $897 million. Available at: http://csdd.tufts.edu/NewsEvents/RecentNews.asp?newsid=29. (Accessed December 2009).
10. Keyhani S, Diener-West M, Powe N. Trends in drug development time and price. Available at: http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=103623139.html. (Accessed December 2009).
11. Meyer RJ. FDA’s Drug Approval Process. Available at: www.hhs.gov/asl/testify/t040401.html. (Accessed December 2009).
12. Psychiatric news. Did delay of ephedra ban cause unnecessary deaths? Available at: http://pn.psychiatryonline.org/content/39/3/24.full. (Accessed December 2009).
13. Casewatch. Utah appeals court upholds FDA ephedra ban. Available at: www.casewatch.org/fda/court/ephedra/utah2.shtml. (Accessed December 2009).
14. FTC. Multilevel Marketing Plans. Available at: www.ftc.gov/bcp/edu/pubs/consumer/invest/inv12.shtm. (Accessed December 2009).
15. Attn: optometrists and ophthalmologists. Available at: www.easypracticeprofits.com. (Accessed December 2009).
16. Home Remedies for You. Conjuctivitis. Available at: www.home-remedies-for-you.com/remedy/Conjunctivitis.html. (Accessed Decemember 2009).
17. Kanski JJ. Clinical Ophthalmology: A Systematic Approach, 3rd ed. Oxford. Butterworth-Heinemann. 1994:77.
18. Natural Eye Care. Conjunctivitis. Available at: www.naturaleyecare.com/diseases.asp?d_num=3. (Accessed December 2009).
19. Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001211.
20. Allergies: How to avoid them. Available at: www.naturalways.com/alergy1.htm. (Accessed December 2009).
21. Dunstan JA, Breckler L, Hale K, et al. Supplementation with vitamins C, E, beta-carotene and selenium has no effect on anti-oxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy. 2007 Feb;37(2):180-7.
22. Barret S. Gastrointestinal quackery: colonics, laxatives and more. Available at: www.quackwatch.com/01QuackeryRelatedTopics/gastro.html. (Accessed December 2009).
23. Miljanovic B, Trivedi KA, Dana MR, et al. Relation between dietary N-3 and N-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005 Oct;82(4):887-93.
24. FDA. Dietary supplement current good manufacturing practices (CGMPs) and interim final rule (IFR) facts. Available at: www.fda.gov/Food/DietarySupplements/GuidanceComplianceRegulatoryInformation/RegulationsLaws/ucm110858.htm. (Accessed December 2009).
25. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome. A Delphi approach to treatment recommendations. Cornea. 2006 Sep;25(8):900-7.
26. Dry Eye Workshop (DEWS) Committee. 2007 Report of the Dry Eye Workshop (DEWS). Ocul Surf. 2007 Apr;5(2):65-204.
27. Molzon J. The Common Technical Document: the changing face of the New Drug Application. Available at: www.nature.com/nrd/jour
nal/v2/n1/fig_tab/nrd990_F1.html. (Accessed January 2010).


