A Deeper Look at DEWS II Making the Diagnosis with TFOS DEWS II The Tear Film: More Complex Than You May Realize |
The Tear Film and Ocular Surface Society’s second Dry Eye Workshop (DEWS II) report has redefined how we think about dry eye disease (DED) from diagnostic, pathophysiological and management perspectives. This article explores the clinical implications of new findings on the epidemiology of the disease and how they might help us diagnose and manage DED in new or previously diagnosed DED patients.1 Additionally, it will cover how these new findings further expose the impact of the disease from individual and societal perspectives.
International Impact
DED affects tens of millions of individuals around the world, and both its frequency and impact increase with age. Consequently, it is considered a major international health concern. Dry eye is the most commonly reported reason for seeking medical eye care, and up to one in five patients presenting to hospital outpatient clinics or optometry practices experience dry eye.2-5
Dry eye has significant socioeconomic implications. In the United States, the average cost of dry eye management is $11,302 per patient and $55 billion overall.6 While absolute costs vary depending on health care systems and insurance plans, it is clear that DED results in increased healthcare usage and cost. With increased life expectancy and an aging population, the economic and social impacts of this condition are expected to grow substantially.
The most significant societal impact occurs through reduced productivity at work, as well as reduced vision and quality of life for affected individuals.7,8 Those with dry eye are two to three times more likely to report problems with everyday activities such as reading, performing professional work tasks, computer use, watching television and daytime or nighttime driving.9-11
As practitioners, we generally look to optimize static visual acuity, but for our dry eye patients, quality of dynamic vision is compromised. As such, we need to tailor our questioning to focus on the specifics of the visual issue, its impact and its changes with treatment. New technologies such as real-time, smartphone-based data capture capabilities may help in our understanding of the impact of dry eye on quality of life.
Evaporative is Important
Recent clinical and epidemiological studies have confirmed that most dry eye patients show signs of mixed evaporative and aqueous deficient dry eye, with evaporative dry eye being the most common manifestation.12-15 Evaporative dry eye is most often associated with meibomian gland dysfunction (MGD). For clinicians, this means taking greater care when examining the eyelids and looking for a short tear break-up time (TBUT), even when the tear volume is normal and no dry eye symptoms exist. One recent study showed that asymptomatic MGD is twice as common as symptomatic MGD, while symptomatic MGD is equally as common in men and women in a Caucasian population.16
Another study, based on claims data in the United States, suggests that one of the main risk factors in the development of more severe disease over time is the presence of MGD signs.17 While still an unproven hypothesis, recognizing signs of MGD early and intervening may limit progression to more severe and symptomatic disease.
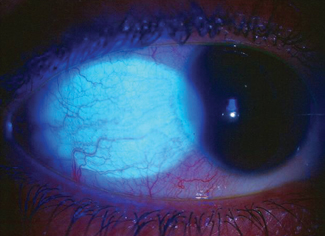 |
| Fig. 1. Allergic conjunctivitis, shown here, is a probable risk factor for DED. Photo: Greg Black, OD, and Julie Tyler, OD |
Prevalence
Determining how common dry eye is depends on how the disease is defined. However, we can make some broad conclusions based on DEWS II:1
- Signs of dry eye, including short TBUT, reduced tear volume, ocular surface staining or lid signs, occur in up to 75% of some populations and are more variable than disease prevalence based on reporting of symptoms.18 Some signs (e.g., corneal staining) are non-specific and may reflect secondary outcomes of disease or be a consequence of normal aging. The DEWS II diagnostic methodology report describes a pathological cut-off as ±2 standard deviations from the mean in a normal age-matched population, which may help us interpret these findings and apply them in our practice settings.18
- The prevalence of DED increases with age, ranging from a 2% increase in prevalence per decade for practitioner-diagnosed dry eye to 10% per decade for disease based on low tear volume. Intriguingly, studies in school children and young adults show unexpectedly high rates of dry eye, perhaps raising the importance of exploring potential risk factors such as digital device use in these populations.
- Women generally have a higher rate of disease than men, although differences between the sexes in prevalence rate become significant with age. There are limited MGD studies stratified by sex and age, and so far the findings for sex are equivocal.
- For disease definitions similar to dry eye, Asian populations have a higher rate than those in Caucasian populations. The reasons for this are unclear, but they may include genetic or environmental determinants. Techniques such as spatial epidemiology and geographic mapping may help us to understand the impact of broad environmental differences such as climate, altitude, air quality, population density and other human development indices.
- Severe dry eye with major impact on quality of life can affect up to 10% of the population over 50.
Who and Why?
In DEWS II, risk factors are based on how robust the association with dry eye is (consistent, probable or inconclusive) and whether the risk factor is modifiable or non-modifiable. Risk factors are important in patient education, framing triaging questions and directing management strategies such as environmental modification, dietary changes or management of MGD.
Non-modifiable risk factors include age, sex, MGD, Sjögren’s syndrome and connective tissue diseases.
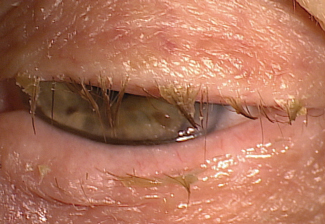 |
| Fig. 2. A history of blepharitis has been associated with dry eye’s worsening visual symptoms. Photo: Paul Karpecki, OD |
Consistent modifiable factors, meanwhile, include androgen deficiency, computer use, certain environmental conditions, contact lens wear, hormone replacement therapy and common medications such as beta-blockers, anxiolytics, antidepressants, cholinergic agonists, isotretinoin and diuretics.
Probable factors are diabetes, thyroid disease, viral infections, allergic conjunctivitis (Figure 1), low fatty acid intake and refractive surgery.
A limited number of high-quality studies have looked at factors that predispose patients to more severe disease. One study looked at characteristics associated with worsening dryness symptoms, and the researchers found that more money spent on treatment, a history of severe symptoms and use of systemic beta-blockers were predictors.17 The study also concluded that worsening vision symptoms were associated with a history of ocular surgery, depression and MGD or blepharitis (Figure 2).17
A greater emphasis on understanding the natural history of both treated and untreated disease could help us better identify patients likely to progress to more severe disease and learn what benefits early intervention might provide.
The Role of Genes
Genetic susceptibility is believed to be important in the development of DED, but because of its complexity and heterogeneity, little research has been done in this area. Heritability in disorders or traits is often measured in studies of monozygotic and dizygotic twins, which allows us to separate genetic effects, shared environmental effects (events that happen to both twins) and unique environmental effects (events or situations experienced by one twin but not the other). Heritability is measured on a zero to one scale and tells us what proportion of variation in a condition or trait is attributed to genetic factors.
Dry eye signs and symptoms showed moderate heritability (0.3) in a twin study from the United Kingdom (Table 1).19 Interestingly, blepharitis (anterior and posterior) had a strong heritability (0.78), whereas TBUT was entirely attributed to environmental effects and showed no evidence of a genetic contribution. By way of comparison, other studies have shown higher levels of heritability for other ocular conditions such age-related macular degeneration or increased intraocular pressure.20 A better understanding these contributions would likely help us to appropriately educate patients and their family members.
Genome-wide studies, in which DNA is collected and analyzed, identify relevant genetic variants in in single nucleotide polymorphism. While conditions such as myopia or glaucoma have been reasonably well characterized in these studies, a broader spectrum of dry eye has yet to be addressed and should be a focus for future studies.
| Table 1. Genetic and Environmental Contributions to Different DED Outcome Variables19 0–1 scale, 95% confidence intervals | |||
| Genetic Effects | Shared Environmental Effects | Unique Environmental Effects | |
| Dry eye symptoms within the last three months | 0.29 (0.18-0.40) | 0.71 (0.60-0.82) | |
| Dry eye diagnosis and current use of artificial tears | 0.41 (0.26-0.56) | 0.59 (0.58-0.93) | |
| Interblink interval (seconds) | 0.25 (0.07-0.42) | 0.75 (0.58-0.93) | |
| Tear osmolarity (mOsmol/L) | 0.40 (0.25-0.53) | 0.60 (0.47-0.75) | |
| Schirmer 1 (mm/5 minutes) | 0.58 (0.43-0.70) | 0.42 (0.30-0.57) | |
| Fluorescein tear break-up time (seconds) | — | 0.30 (0.18-0.40) | 0.71 (0.60-0.82) |
| Signs of anterior or posterior blepharitis | 0.78 (0.59-0.90) | 0.22 (0.10-0.41) | |
What's Next?
Since the publication of the original TFOS DEWS report, there has been considerable focus on the epidemiology of DED. Despite this, we have seen limited data from regions south of the equator, so large regions of the world are not represented in our current understanding of dry eye prevalence and risk factors. It has been well established that dry eye is common, has a significant impact on patients and has a considerable (and growing) societal cost due to reduced quality of life and reduced work productivity.
Our current understanding of DED risk factors helps us triage our patients and suggest possible management strategies. To better serve our patients, however, we need a more comprehensive understanding of how common the varying severities of dry eye are and the impact of current technologies, such as mobile devices, on the disease. Some evidence from studies in Asia suggests that youth is not protective for dry eye to the extent we would anticipate, but few studies have looked at populations younger than 40. This is clearly an area in need of further study.
Other risk factors such as the impact of climate, environmental and socioeconomic factors also deserve further study. Lastly, and probably most important for ODs, we need to determine whether our management strategies work so that we can modulate signs and symptoms in a predictable way with intervention. Through a better understanding of the natural history of treated and untreated disease, we can predict and better manage patients who will progress to more severe disease. Let’s hope we don’t have to wait another 20 years for DEWS III to provide the answers.
Dr. Stapleton is a professor in the School of Optometry and Vision Science at the University of New South Wales. Her research interests are in epidemiology of contact lens-related infection. She was the chair of the TFOS DEWS II epidemiology subcommittee.
1. Stapleton FJ, Alves M, Bunya V, et al. TFOS DEWS II epidemiology report. Ocular Surf. 2017;15(3):334-365. |


