The search for improved comfort drives innovation and development of improved contact lens modalities—glass lenses gave way to plastic materials, and corneal lenses replaced older, molded scleral designs. But, it is clear that safe and successful contact lens wear requires more than just permeability. Practitioners now recognize that a lifetime of successful contact lens wear requires clean lens surfaces with sustained wettability and biocompatibility.
Physiology, Safety and Comfort
The first soft lenses, introduced by Bausch & Lomb in 1971, were far more comfortable than the PMMA hard lenses that were available then. Still, even with the advent of soft lenses, comfort continued to be a problem until the early 1980s, when advances in lens materials and designs provided moderate improvement in patient comfort. Despite the advances, additional comfort and safety issues loomed on the not-too-distant horizon.
The 1980s was the decade of extended-wear contact lenses—the modality was convenient, and thanks to material advances, comfortable. However, complications, including microbial keratitis (MK), became increasingly common and eventually caught the attention of the public media. A decisive moment in the modern history of contact lenses occurred when studies comparing the incidence of MK in daily and extended-wear lenses were published in the New England Journal of Medicine in 1989. Overnight extended wear was found to have a five-times greater risk of MK vs. conventional daily wear.1 The resultant scientific push to find both a cause and a solution for these relatively high rates of MK implicated insufficient corneal oxygen levels as a primary cause of MK.2
In 1999, after extensive and prolonged research, the oxygen permeability levels needed to meet the projected minimal oxygen requirements were finally achieved by Bausch & Lomb with the introduction of their Purevision silicone hydrogel.3 Highly permeable materials reduced levels of edema that occurred from overnight wear and appeared to minimize longer-term corneal changes (e.g., epithelial and endothelial changes), limbal redness and neovascular response.4
Increasing available oxygen to the cornea was believed to be the key solution to the high rates of MK reported in the 1989 study, and subsequently by another group of researchers in 1999.5 Both groups found MK rates of approximately 20 per 10,000 annually in overnight extended wear—about five-times the rate observed with soft lens daily wear.1,5 With the advent of silicone hydrogel materials featuring oxygen permeability of over 100Dk, many believed that the risk of MK associated with overnight wear would diminish. Contrary to this prediction, a reduction in infection rates was not observed in U.S. Food and Drug Administration-mandated post-approval surveillance studies.6 More recent studies found that the rate of infection in extended wear was essentially unchanged from the earlier reports—slightly more than 20 per 10,000 in extended wear and 4 per 10,000 in daily wear annually.7,8
Despite the absence of a significant reduction in risk as well as evidence of increases in rates of other complications, such as infiltrative keratitis, the improved corneal physiology of silicone hydrogel materials has helped drive their rapid adoption. However, the complex chemistry of these materials has made it much more difficult to maintain clean and wet contact lens surfaces. This, in addition to the higher modulus of some of these materials, has refocused attention on patient comfort issues.
Polymer Chemistry
Silicone hydrogel materials derive from the marriage of polymers traditionally used for soft hydrophilic materials (currently termed conventional hydrogels) and hydrophobic polymers that previously served as the base for gas-permeable contact lens materials. These materials were initially classified with the traditional hydrophilic groups developed in the 1980s as groups 1 and 3 contact lens materials. It quickly became apparent, however, that these novel materials behave quite differently from earlier hydrogels. As a result, they are now recognized as a new class—group 58. When comparing silicone hydrogels and conventional hydrogels, research has shown that they differ in their interaction with the environment, that they absorb more lipids from the tears and that they absorb lower levels of the tear proteins.9,10 Silicone hydrogels also have different affinities for certain care system preservatives.11-14
Advances in lens care product formulations have also added to the complexity and potential for interaction between materials and lens care components. This has been observed in increased corneal staining and related comfort issues associated with combinations of lens care products and lenses.15,16 The complexity of modern lenses and lens care products has made it critical that practitioners who prescribe these products consider the effects of their combined use.
Lens Surfaces and Comfort
Combining plastic and water to create a hydrogel material was a remarkable scientific breakthrough, accomplished by Czech scientists Otto Wichterle and Drahoslav Lim in the late 1950s. Beyond his genius as a chemist, Dr. Wichterle envisioned the practical use of hydrogel materials to make contact lenses and actually created prototype lenses. Because hydrophilic lenses contain water and are constantly in a wet environment, it was generally assumed that the water content of the lens remained stable after equilibrating when taken from the storage medium and placed within the ocular environment.17 However, even with high water-content lenses, and in some cases even more so, comfort remained an issue for many patients.
The ocular environment is complex and can easily become destabilized. A lens within the ocular environment can have a profound effect on the stability of the environment and comfort of the patient. The impact of contact lens wear on the ocular surface includes more rapid fluorescein break-up time, increased corneal staining, and frank damage to the wiper surfaces of the lid. This has been described as lid wiper epitheliopathy and is associated with diminished lens comfort.18
Contact lens drop-out is a problem for both practitioners and industry alike. Industry data show that every year, approximately the same number of patients discontinue contact lens wear as those who become new wearers.19 Clinical experience has shown that discomfort, particularly end-of-day discomfort and dryness, are primary causes of discontinuation of lens wear. It is incumbent on us as eye care practitioners to understand the causes and develop effective approaches to minimizing contact lens drop-out rates. Understanding contact lens-related discomfort requires a thorough knowledge of contact lens materials and their interaction with the eye.
To gain insight into what occurs during contact lens wear, it is important to first understand how a normal ocular surface maintains a comfortable environment. This knowledge has been facilitated by a significant amount of research regarding dry eye and tear dysfunction. Much of this data is summarized in the 2007 Report of the Dry Eye WorkShop.20
There is an increased incidence of dryness when the inter-blink interval is greater than the fluorescein break-up time.21 This function has been termed the Ocular Protection Index (OPI). Although somewhat variable, a normal patient shows tear film break-up times (TFBUT) in excess of 10 to 12 seconds. Normal blink intervals are generally around seven seconds, so normal patients do not experience tear break-up on the ocular surface and generally remain comfortable. Contact lens wearers experience more rapid TFBUT: typically less than four seconds. As a result, the front surface of the lens dries during each of the nearly 7,000 blinks a day.
During each blink, the lids, especially the superior lid, sweep across the cornea. This process is necessary to wipe and resurface the cornea. A study of dry eye patients found that damage to the specialized stratified squamous epithelial wiping surfaces of the lids can be observed after staining with fluorescein and rose bengal.18 Findings demonstrate that 76% of patients exhibiting dry eye symptoms showed staining of the lid wiper vs. 12% of patients who did not report dry eye symptoms. And, the degree of staining in the symptomatic patients correlates to severity of symptoms.18,22 The correlation between reported contact lens dryness and the observation of lid wiper epitheliopathy was strong (see “Incidence of Lid Wiper Staining in Contact Lens Wearers”).22
Incidence of Lid Wiper Staining in Contact Lens Wearers22 Asymptomatic Wearers (n=75) Symptomatic Wearers (n=30)
No Staining
Grade 1
Grade 2
Grade 3
84%
11%
3%
3%
27%
37%
20%
17%
Contact Lens vs. Eye
Is the contact lens surface different from the ocular surface? A critical question: How can the surface of the contact lens and its interaction with the lid be modified to make lenses more comfortable throughout the wearing day? Beyond the physical effects of the rigidity of the lens and edges resulting from material properties and design, changes in the lens surface are critical if we hope to improve the all-day comfort of lenses.
In soft lens wearers, tear break-up on the front surface of the lens has been observed to occur much more quickly than it would occur on the cornea itself.23 Exposing the unwetted lens surface to the environment is believed to cause the lens to dry. But, lens materials are homogenous, and with conventional hydrogel materials containing as much as 70% water, drying seemed improbable, especially with the eye refreshing the tear film at every blink.
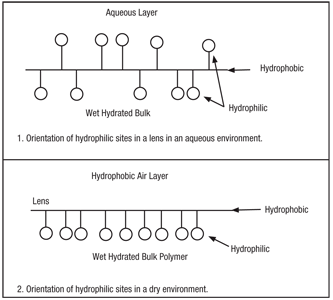 Careful examination of the structure of the lens polymers reveals that all lens materials, even conventional high water-content hydrogels, have sites in the polymer that are hydrophilic (“water loving”) and sections that are hydrophobic (“water hating”). In the base state, bathed in water, both the hydrophilic site at the surface and in the bulk are oriented to attract water and make the overall structure and surface hydrophilic (figure 1). When the lens is removed from an aqueous environment and is in air, there is a shift in the overall arrangement of the hydrophilic and hydrophobic groups in the polymer—air is hydrophobic. As the surface dries, the hydrophilic group looks for an environment satisfying its need for water, and the polymer structure rotates so that the hydrophilic group finds a water loving environment in the inside or bulk of the polymer. The opposite occurs at the surface (figure 2).
Careful examination of the structure of the lens polymers reveals that all lens materials, even conventional high water-content hydrogels, have sites in the polymer that are hydrophilic (“water loving”) and sections that are hydrophobic (“water hating”). In the base state, bathed in water, both the hydrophilic site at the surface and in the bulk are oriented to attract water and make the overall structure and surface hydrophilic (figure 1). When the lens is removed from an aqueous environment and is in air, there is a shift in the overall arrangement of the hydrophilic and hydrophobic groups in the polymer—air is hydrophobic. As the surface dries, the hydrophilic group looks for an environment satisfying its need for water, and the polymer structure rotates so that the hydrophilic group finds a water loving environment in the inside or bulk of the polymer. The opposite occurs at the surface (figure 2).
In practice, we see this phenomenon as a contact lens is worn in the eye. The lens surface dries over time, and this can be readily observed as a change in surface wettability. Surface wettability is a function of the contact angle formed between the surface and a liquid droplet. The contact angle is defined as the angle formed by the solid surface and the tangent line to the upper (droplet) surface at the end point. It can be measured in a variety of ways.23-27
A study examining the surface wettability of lenses under systems designed to model this change in polymer orientation found that conventional lenses equilibrated in saline over time showed advancing surface contact angles that demonstrate the full hydration and a hydrated surface.23 By exposing the lenses to cycles involving 1.5 minutes of drying followed by five minutes in saline, the contact angles varied from 25° in etafilcon lenses coming directly from the package to nearly 100° in 10 simulated cycles, thus showing the effect of the drying process. What is more interesting is that once it occured, this process was not easily reversible.23
When this technique is applied to lenses over a normal wear cycle, the findings demonstrate the same effect. In measurement of ex vivo lenses after eight hours of wear, the contact angle was measured at 105° to 118°. Compare this to the 100° measurement obtained in the in vitro model after 10 simulated cycles.23,24 At the start of the measurement with a lens taken directly from its packaging solution, the advancing contact angle was 25°.28
When these same studies are applied to silicone hydrogel lens materials, the baseline wetting as measured by advancing contact angles reveals the effectiveness of different approaches to making the surface wettable. The plasma-bonded chemical treatment in the Night and Day lens (CIBA Vision) shows superior surface wetting vs. attempts to modify the surface using an internal wetting agent (see “Effects of Surface Treatment of Silicone Hydrogel Lenses”).25
Etafilcon A
Effect of Surface Treatment of Silicone Hydrogel Lenses
Brand
Material
Advancing Contact Angle Directly
from Package
Advancing
Contact Angle after Eight Simulated Blink Cycles (Five Minutes in
Saline and 1.5 minutes
in air)
O2Optix
Lotrafilcon B
30º
60º
Focus Night and Day
Lotrafilcon A
50º
50º
Acuvue Advance
Galyfilcon A
100º
100º
Acuvue 2 (Traditional Hydrogel)
70º
110º
The second set of measurements relates to the estimate of the effect of lubrication achieved during each blink. Measuring the coefficient of friction provides an estimate of the drag upon the lid during each blink. The coefficient of friction describes resistance of two surfaces moving across each other. It is independent on both the force pushing the two surfaces together and the direction of the movement, whether horizontal or vertical.
A study that analyzed the effect of interactions between tissues in an attempt to understand the effect on the lid in dry eye used tissue from human umbilical vein segments in a reciprocating pin-on-disc friction test device.29 The coefficient of friction was affected by the wetness of the surfaces, and various demulcent formulations reduced the coefficient of friction. An in vitro procedure for evaluation of lens-on-tissue friction proposed by the lead researcher of this study now uses tissue from bovine pericardium, a reproducible material made under controlled conditions.30
These experiments demonstrate the importance of filling the interface with the “right” material and achieving appropriate properties to minimize the coefficient of friction. Using microscopy to evaluate these initial experiments with tissue-on-tissue friction shows the role of even small changes in friction and damage to the tissue surface.
Measurements of wettability of the lens surface and of surface lubrication are two approaches that provide in vitro and ex vivo measurements of key material and solution properties that affect contact lens comfort. Correlation of data from these measurements, as well as clinical effects upon lid wiper surfaces provide us with the tools to understand the surfaces of contact lenses and how to provide more comfortable lens wear throughout the day.
Newer contact lens designs and lens care products incorporate components that increase surface wettability and decrease friction to improve and sustain comfort. Examples of lenses optimized for more wettable surfaces include Dailies AquaComfort PLUS (CIBA Vision) and 1-Day Acuvue Moist (Vistakon). Currently available lens care products incorporating enhanced wetting agents include OPTI-Free RepleniSH (Alcon) and AQuify MultiPurpose Solution (CIBA Vision). Additional products containing innovative wetting agents will be introduced in 2010.
Performance and Comfort
Contact lenses have advanced dramatically from their genesis as uncomfortable refractive devices, offering only limited wearing time. But, keeping pace with patient expectations and demands has been challenging. Today’s patients are far more interested in immediate and sustained comfort than they are intrigued by the novelty of contact lens wear. Patients expect good comfort and stable vision for at least 12 to 14 hours a day, and with a greater variety of available refractive options than ever, they are likely to seek alternatives if their contact lenses fail to meet their expectations. New technologies for lenses and lens care require continuing evaluation of the paradigms for in vitro and in vivo testing to meet the comfort expectations of patients.
Awareness of patient expectations and new technologies will help ensure that contact lenses remain a viable choice for many years to come. And, understanding the complex technical issues is becoming a requisite for maximizing the patient experience.
Researchers have the primary tools for the development of new approaches to improved contact lens surfaces. Meeting our patients’ comfort expectations comes directly from continued advances in surface chemistry—either chemically modifying the surface, adsorbing or bonding long-lasting wetting components to the surface during storage or care, or providing an internal wetting system that releases or migrates to the surface during wear. The future of contact lenses will undoubtedly lead to new products that combine excellent visual performance and long-lasting, sustained comfort.
Dr. Epstein is a founding partner of North Shore Contact Lens & Vision Consultants, P.C., a referral-based contact lens specialty and primary care practice in Roslyn Heights, N.Y. He is also a consultant for Alcon. Dr. Stone retired from Alcon Laboratories as Vice President for Consumer Products Research and Development in 2006 after 25 years in the eye care industry working for Bausch & Lomb and Wesley-Jessen Corp, in addition to Alcon. He developed many of the care systems used in the field.
1. Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended soft contact lens wearers. N Engl J Med. 1989 Sep 21;321(12):779-83.
2. Weissman BA, Mondino BJ. Risk factors for contact lens associated microbial keratitis. Cont Lens Anterior Eye. 2002 Mar;25(1):3-9
3. Holden BA, Merz GW. Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Invest Ophthalmol Vis Sci. 1984 Oct;25(10):1161-7.
4. Bergenske P, Long B, Dillehay S, et al. Long-term clinical results: 3 years of up to 30-night continuous wear of lotrafilcon A silicone hydrogel and daily wear of low-Dk/t hydrogel lenses. Eye Contact Lens. 2007 Mar;33(2):74-80.
5. Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact lens associated microbial keratitis and its related morbidity. Lancet. 1999 Jul 17;354(9174):181-5.
6. Schein OD, McNally JJ, Katz J, et. al. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005 Dec;112(12):2172-9.
7. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008 Oct;115(10):1655-62..
8. Dart JK, Radford CF, Minassian D. et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008 Oct;115(10):1647-54.
9. Jones L, Senchyna M, Glasier MA, et al. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens. 2003 Jan;29(1 Suppl):S75-9.
10. Senchyna M, Jones L, Louie D, et al. Quantitative and conformational characterization of Lysozyme depositied on Balafilcon and Etafilcon contact lens materials. Curr Eye Res. 2004 Jan;28(1):25-36.
11. Rosenthal RA, McDonald MM, Schlitzer RL, et al. Loss of bacterial activity from contact lens storage solutions. CLAO J. 1997 Jan;23(1):57-62.
12. Dannelly HK, Waworuntu RV. Effectiveness of contact lens disinfectants after lens storage. Eye Contact Lens. 2004 Jul;30(3):163-5.
13. Rosenthal RA, Dassanayake NL, Schlitzer RL, et al. Biocide uptake in contact lenses and loss of fungal activity during storage of contact lenses. Eye Contact Lens. 2006 Dec;32(6):262-6.
14. Warburton K, Noble-Wang JA, Henry BM, et al. Absorption of alexidine by contact lenses and lens cases and its effect on disinfection activity against Fusarium solani. Poster Q-426 presented at American Society of Microbiology. Toronto. 2007.
15. Jones L, MacDougall N, Sorbara GL. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci. 2002 Dec;79(12):753-61.
16. Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses and multipurpose solution combinations. Optometry. 2008 Aug;79(8):444-54.
17. Efron N, Brennan NA, Bruce AS, et al. Dehydration of hydrogel lenses under normal wearing Conditions. CLAO J. 1987 May-Jun;13(3):152-6.
18. Korb DR, Herman JV, Finnemore HE, et al. Lid wiper epitheliopathy and associated dry eye symptoms. IOVS 2004; 45 E-Abstract 100.
19. Bart JT. 2004 Contact Lens Spectrum Annual Report. Cont Lens Spectrum. 2005 Jan;20(1).
20. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):179-93.
21. Ousler III GW, Hagberg KW, Schindelair M, et al. The Ocular Protection Index. Cornea. 2008 Jun;27(5):509-13.
22. Korb DR, Greiner JV, Herman JP, et al. Lid-wiper epitheliopathy and dry eye symptoms in contact lens wearers. CLAO J. 2002 Oct;28(4):211-6.
23. Ketelson HA, Meadows D. Wettability of soft contact lenses new thoughts about an old problem. Rev Cornea & Cont Lens. 2004 Oct;141(7):34-8.
24. Ketelson HA, Meadows DM, McQueen N, Stone RP. Enhancing wettability with multi-purpose solutions. Rev Cornea & Cont Lens. 2005 Jan/Feb;142(1);44-7.
25. Ketelson HA, McQueen N, Meadows D, Stone RP. Wettability of silicone hydrogel lenses in the presence of tear components. Rev Cornea & Cont Lens. 2005 Apr;142(3):24-8.
26. Tonge S, Jones L, Goodall S, Tighe B. Ex vivo wettability of soft contact lenses. Curr Eye Res. 2001 Jul;23(1):51-9.
27. Moldanado-Codina C, Efron N. Dynamic wettability of pHEMA-based hydrogel contact lenses. Ophthalmic Physiol Opt. 2006 Jul;26(4):408-18.
28. Alonso-Caneiro D, Iskander DR, Collins MJ. Tear film surface quality with soft contact lenses using dynamic-area high-speed videokeratoscopy. Eye Contact Lens. 2009 Sep;35(5):227-31.
29. Meyer AE, Baier RE, Chen H, et al. Differential tissue-on-tissue lubrication by ophthalmic formulations. J Biomed Mater Res B Appl Biomater. 2007 Jul;82(1):74-88.
30. Meyer AE. An in-vitro procedure for evaluation of lens-on-tissue friction. Available at:
www.lsu-eye.lsuhsc.edu/docs/Meyer%20Talk%2011-08.pdf.


